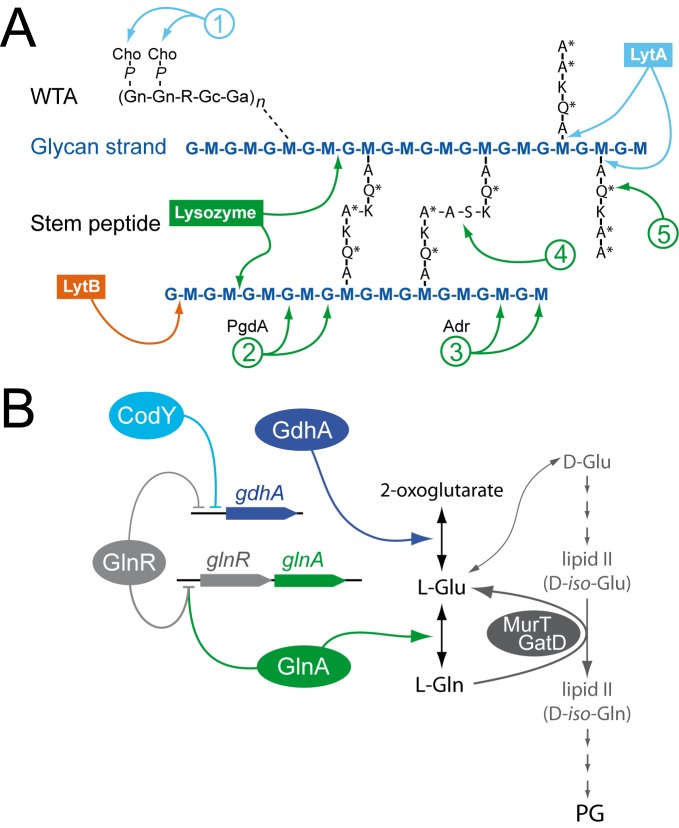
Fig 5. Pneumococcal cell wall composition, documented alterations and potential connections with GlnR-CodY through Glu/Gln metabolism.
(A) Diagram of pneumococcal cell-wall composition, with LytA, LytB and lysozyme cleavage sites, as well as cell-wall alterations known to affect hydrolytic activities. The glycan chains in pneumococcal PG are made of alternating MurNAc (M) and GlcNAc (G) residues. WTA is composed of repeating units containing two N-acetylgalactosamine residues (Gn), ribitol phosphate (R), glucose (Gc) and 2-acetamido-4-amino-2,4,6-trideoxygalactose (Ga). WTA chains are linked to PG via an unknown linkage (dotted line). Each R moiety can further be alanylated, although an initially proposed a-GalpNAc substitution [48] could not be confirmed in a later work [32]. The amino acids in the stem peptide are designated in single-letter code, with an asterisk indicating d-configuration. Modifications in the cell wall are indicated by numbers: (1) decoration of WTA by phosphocholine (P-Cho); (2) deacetylation of G by PgdA; (3) O-acetylation of M by Adr; (4) Branching between muropeptides by MurMN; (5) Amidation at 2nd stem peptide residue by MurT/GatD. (B) GlnR and CodY in the regulation of Gln/Glu metabolism of S. pneumoniae. Both regulators directly repress gdhA, involved in Glu synthesis. GlnR also represses glnA, involved in Gln synthesis, with DNA binding of GlnR to DNA shown to depend on GlnA [13]. Gln acts as a donor for amidation of the 2nd stem peptide residue (see panel A) catalyzed by the MurT/GatD amidotransferase.