Abstract
Experimental studies in preclinical mouse models of breast cancer have shown that chronic restraint stress can enhance disease progression by increasing catecholamine levels and subsequent signaling of β-adrenergic receptors. Catecholamines also signal α-adrenergic receptors, and greater α-adrenergic signaling has been shown to promote breast cancer in vitro and in vivo. However, antagonism of α-adrenergic receptors can result in elevated catecholamine levels, which may increase β-adrenergic signaling, because pre-synaptic α2-adrenergic receptors mediate an autoinhibition of sympathetic transmission. Given these findings, we examined the effect of α-adrenergic blockade on breast cancer progression under non-stress and stress conditions (chronic restraint) in an orthotopic mouse model with MDA-MB-231HM cells. Chronic restraint increased primary tumor growth and metastasis to distant tissues as expected, and non-selective α-adrenergic blockade by phentolamine significantly inhibited those effects. However, under non-stress conditions, phentolamine increased primary tumor size and distant metastasis. Sympatho-neural gene expression for catecholamine biosynthesis enzymes was elevated by phentolamine under non-stress conditions, and the non-selective β-blocker propranolol inhibited the effect of phentolamine on breast cancer progression. Selective α2-adrenergic blockade by efaroxan also increased primary tumor size and distant metastasis under non-stress conditions, but selective α1-adrenergic blockade by prazosin did not. These results are consistent with the hypothesis that α2-adrenergic signaling can act through an autoreceptor mechanism to inhibit sympathetic catecholamine release and, thus, modulate established effects of β-adrenergic signaling on tumor progression-relevant biology.
Keywords: Restraint stress, sympathetic nervous system, catecholamines, α-adrenergic receptor, breast cancer, metastasis, bioluminescent imaging
1. Introduction
Clinical and epidemiological studies have shown that stress-related psychosocial factors in breast cancer patients can associate with accelerated progression of disease (Antoni et al., 2006; Chida et al., 2008). Experimental studies of stress effects in preclinical mouse models of breast cancer have shown that chronic restraint can increase the rate of primary tumor growth (Thaker et al., 2006) or cause primary tumor to become more metastatic (Sloan et al., 2010). Moreover, the non-selective β-blocker, propranolol, abrogated the effect of chronic restraint on progression, strongly suggesting that stressor-induced catecholamines act through β-adrenergic receptors to mediate the effect of chronic restraint on the tumor microenvironment (Thaker et al., 2006; Sloan et al., 2010).
Catecholamines can also directly promote breast cancer cell proliferation in vitro through α-adrenergic receptors. Several α-adrenergic receptor antagonists blocked the proliferative effect of epinephrine and norepinephrine on human MCF-7 breast cancer cells in vitro (Vázquez et al., 1999). Similarly, an α-adrenergic receptor antagonist blocked the proliferative effect of epinephrine on human MDA-MB-231, human IBH-4, and mouse MC4-L5 breast cancer cells (Pérez Piñero et al., 2012).
Increased α-adrenergic signaling has also been shown to increase breast cancer progression in vivo under non-stress conditions with models that involve subcutaneous injection into the flank (Bruzzone et al., 2008; Bruzzone et al., 2009; Pérez Piñero et al., 2012) and an orthotopic model that exhibits primary mammary tumor and distant metastasis (Szpunar et al., 2013). In the latter orthotopic study (Szpunar et al., 2013), an α2-adrenergic agonist was shown to increase primary tumor growth rate and metastasis using the murine 4T1 cell line, which was found to lack functional α-adrenergic receptors. Thus, that study suggested that catecholamines may also signal host α-adrenergic receptors to promote breast cancer progression through indirect mechanisms.
Multiple reports have shown that pre-synaptic α2-adrenergic receptors can mediate an autoinhibition of sympathetic transmission (Hein et al., 1999; Scheibner et al., 2001; Trendelenburg et al., 2001) and that disruption of this negative feedback signal with α2-adrenergic antagonists or gene inactivation of α2-adrenergic receptors enhances norepinephrine release (Starke, 2001). Thus, it is conceivable that blocking inhibitory α2-autoreceptors might increase sympatho-neural discharge and enhance β-adrenergic signaling with its known downstream pro-tumor consequences for breast cancer progression (Thaker et al., 2006; Sloan et al., 2010; Madden et al., 2011). Increased β-adrenergic signaling has been shown to increase accumulation of tumor-associated macrophages in primary mammary tumor, which increased angiogenesis and the expression of metastasis-promoting genes, including Tgfb, Mmp9, and Vcam1 (Sloan et al., 2010). Moreover, direct β-adrenergic signaling in some breast cancer cell lines can increase expression of cytokines that promote tumor angiogenesis and metastasis, including vascular endothelial growth factor (VEGF) and interleukin-6 (IL-6) (Madden et al., 2011).
We were aware of no experimental in vivo studies that examined the effect of α-adrenergic blockade on breast cancer growing in an orthotopic tumor microenvironment. Given the reported ability of α-adrenergic antagonists to block direct effects of stress-typical catecholamines on breast cancer cell proliferation in vitro and the reported role for α-adrenergic signaling in breast cancer promotion in vivo, we hypothesized that α-adrenergic blockade can inhibit breast cancer progression under non-stress and stress conditions in an orthotopic mouse model of breast cancer. However, given the reported ability of α-adrenergic receptors to mediate an autoinhibition of sympathetic transmission, we also tested the alternate hypothesis that blockade of such α-autoreceptors can promote breast cancer progression under non-stress and stress conditions.
2. Methods
2.1. Animals
Seven-week-old female nu/nu mice (Charles River Laboratories, Crl:NU-Foxn1nu) were housed under BSL2 barrier conditions on an individually ventilated cage (IVC) rack in dual filter disposable cages (Innovive, Inc.), in groups of 3–5 per cage, with corn cob bedding, nesting tissue, and ad libitum access to food and water on a 12:12 light:dark cycle at 22°C. All procedures were carried out under protocols approved by the Institutional Animal Use and Care Committee of the University of California, Los Angeles.
2.2. Orthotopic breast cancer model
The MDA-MB-231HM cell line, a more highly metastatic derivative of the MDA-MB-231 human breast adenocarcinoma cell line (Chang et al., 2008), was transduced with a lentiviral vector containing firefly luciferase (Kaminskas et al., 2013) and cultured in DMEM with GlutaMAX™ and Pyruvate (Life Technologies-Gibco, #10569-044), supplemented with 10% FBS (Atlanta Biologicals, #S11550H), at 37°C, in a 5% CO2 atmosphere. The tumor cells (2 × 105) were injected into the left 4th mammary fat pad. Mammary tumor size was measured using digital calipers, and tumor volume was calculated as (length × width2)/2. The frequency and quantity of metastases were tracked in live mice by repeated noninvasive optical imaging of tumor-specific luciferase activity using the IVIS Lumina II system running Living Image 4.0 software (Caliper Life Sciences). After anesthetization with 2% isoflurane and intravenous injection of 150 mg/kg luciferin, mice were photographed under bright-field illumination and images were overlaid with luminescence data gathered over the maximum exposure period without pixel saturation (0.5–60 seconds). Metastasis was measured by triplicate determination at each time point of total bioluminescence in a region of interest of constant size located distant from the primary tumor (thoracic cavity where metastases appeared). Tissue-specific metastasis was measured ex vivo by bioluminescence immediately after sacrifice on Day 28 or 32 of primary tumor growth.
2.3. Chronic restraint
Mice were randomly assigned to home cage control conditions or 2 h per day restraint for 20 days commencing 5 days before tumor cell inoculation. Mice were restrained in a confined space that prevented them from moving freely but did not press on them (Thaker et al., 2006). This paradigm has been shown to induce stress as shown by neuroendocrine activation (Thaker et al., 2006; Manni et al., 2008) and anxiety-like behaviors (Herman et al., 1994).
2.4. α-adrenergic receptor blockade
Phentolamine hydrochloride (Sigma, #P7547) in PBS vehicle, or an equivalent volume of vehicle alone for placebo in control mice and restraint-only mice, was injected subcutaneously at 5mg/kg daily, commencing 5 days before tumor cell inoculation and continuing to the end of the experiment on Day 28. Two experiments with phentolamine were conducted and included the following groups: (1) Control; (2) Restraint + Placebo; (3) No Restraint + Phentolamine; (4) Restraint + Phentolamine. For each experiment, n = 3 to 5 mice per group. An additional experiment was conducted with phentolamine and the non-selective β-blocker, s-propranolol (#P8688) at 2mg/kg, to determine whether the promoting effects of phentolamine were mediated through β-adrenergic receptors and included the following groups: (1) Control; (2) Phentolamine; (3) Propranolol; (4) Phentolamine + Propranolol (n = 5 mice per group). To examine the effect of selective α-adrenergic receptor blockade under non-stress conditions, an experiment was conducted with the α1-blocker, prazosin hydrochloride (#P7791) at 2mg/kg, and the α2-blocker, efaroxan hydrochloride (#E3263) at 5mg/kg, and included the following groups: (1) Control; (2) Prazosin; (3) Efaroxan (n = 4 to 6 mice per group).
2.5. In vitro proliferation assays
Potential direct effects of phentolamine on MDA-MB-231HM proliferation in the absence and presence of catecholamines were tested by treating cells in vitro with phentolamine (0.0001 to 1 μM) and stressor-induced concentrations (Koolhaas et al., 2011) of epinephrine (0.0001, 0.001, and 0.01 μM; Sigma, #E4375) or norepinephrine (0.001, 0.01, and 0.1 μM; Sigma, #A9512). Proliferation was assayed by one-step MTS colorimetric assay (Promega, #G3582) at 24, 48, and 72 h after treatment initiation with a Thermo Scientific Multiskan MCC/340 microplate reader. Treatment media was replaced daily (DMEM with GlutaMAX, Pyruvate, 10% FBS). In each of two or three independent experiments, triplicate wells were prepared for each condition at each time point with initial seeding of 5000 cells per well.
2.6. Sympatho-adrenomedullary and sympatho-neural gene expression
To quantify gene expression of the key biosynthetic enzymes for catecholamine production in the adrenal medulla and sympathetic ganglia, total RNA was extracted from adrenal glands and from bi-lateral sympathetic chains of the thoracic T1-T10 vertebrae (Qiagen RNeasy Mini Kits #74104 and #74704, respectively), with contaminating DNA cleared by on-column DNase digestion (Qiagen RNase-Free DNase Set, #79254). Resulting RNA was assayed by quantitative one-step RT-PCR (Qiagen Quantitect Probe RT-PCR, #204443) using sequence-specific primer-probe sets for biosynthetic enzymes in the adrenal medulla, i.e., tyrosine hydroxylase (Th), dopamine β-hydroxylase (Dbh), and phenylethanolamine N-methyltransferase (Pnmt), and in the sympathetic chain ganglia, i.e., Th, Dbh. (Invitrogen-Applied Biosystems TaqMan Gene Expression Assays; Th, #Mm00447557_m1; Dbh, #Mm00460472_m1; Pnmt, #Mm00476993_m1). Transcripts for the catecholamine-degrading enzymes, monoamine oxidase A, Maoa, and, monoamine oxidase B, Maob, were also assayed (Maoa, #Mm00558004_m1; Maob, #Mm00555412_m1). RT-PCR was completed for tissue samples using triplicate determinations for each gene in each sample according to the manufacturer’s specified protocol on a Bio-Rad iCycler Real-Time Detection System, with enzyme threshold cycle values normalized to values of β-actin mRNA amplified in parallel (Actb, #Mm00607939_s1).
2.7. Statistical analysis
Data are presented as mean ± SE, and all statistical analyses were carried out using SPSS software (v.18). All distributions were examined for outliers and non-normality, and log transformations were applied to normalize distributions where necessary. Mixed-effects linear models (McCulloch et al., 2008) were used to analyze datasets pooled across experiments, where pharmacologic modification of longitudinal trajectory of breast cancer progression in vivo, or proliferation in vitro, was assessed by examining the group x time interaction term (i.e., restraint × phentolamine x day, catecholamine x phentolamine x day, or phentolamine x propranolol x day) and the group main effect alongside preplanned contrast analyses using the t-distribution. Composite catecholaminergic gene expression in the adrenal gland and in the sympathetic ganglia was assessed by examining the restraint × treatment interaction term for cumulative Th, Dbh, and Pnmt gene expression; individual gene expression was analyzed for each tissue site with preplanned contrast analyses in the context of a univariate analysis of variance.
3. Results
3.1. Effects of α-adrenergic blockade on breast cancer progression under non-stress and stress conditions
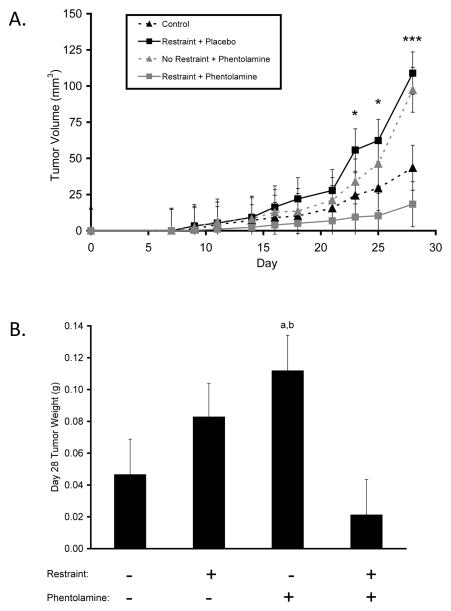
To determine whether α-adrenergic blockade can inhibit breast cancer progression and attenuate the effect of chronic restraint stress on breast cancer progression, we measured the progression of MDA-MB-231HM human breast cancer in the mammary gland for 28 days and used in vivo optical imaging to quantify metastasis of the luciferase-tagged cells from the primary tumor. Chronic restraint increased the growth rate of primary tumor by 2.5-fold versus controls (P = 0.025; Fig. 1A). α-adrenergic blockade with phentolamine inhibited the effect of chronic restraint, as mean primary tumor volume in the chronic restraint plus α-adrenergic blockade group was not significantly different from controls (P values > 0.073; Fig. 1A). However, treatment with phentolamine in the absence of stress significantly increased primary tumor size compared to controls (P values < 0.05; Fig. 1A). Analysis of tumor weight on Day 28 of primary tumor growth showed a similar pattern of results among groups (P values < 0.05; Fig. 1B).
Figure 1.
Effect of α-adrenergic blockade on breast tumor growth under non-stress and stress conditions. (A) Primary tumor volume was measured over 28 days, * P < 0.05, *** P < 0.001 for group effect. (B) Group differences in primary tumor weight on Day 28, a: P < .05 vs. Control, b: P < .01 vs. Restraint+Phentolamine.
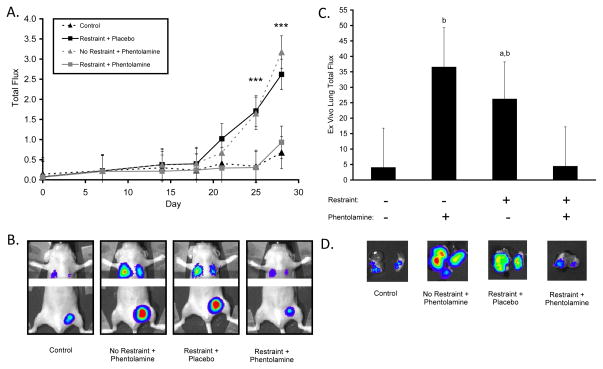
Metastasis development correlated with primary tumor growth (Spearman correlation coefficient, r = 0.76, P < 0.0001). Chronic restraint significantly increased metastasis of primary breast tumor cells to distant tissues by an average of 4.5-fold versus controls (P values < 0.001; Fig. 2A–B). The effect of chronic restraint was inhibited by α-adrenergic blockade, as metastasis values in the chronic restraint plus α-adrenergic blockade group were not significantly different from controls (P values > 0.714; Fig. 2A–B). However, α-adrenergic blockade in the absence of stress significantly increased metastasis levels compared to levels in control mice (P values < 0.01; Fig. 2A). Ex vivo analysis of tumor bioluminescence in lungs confirmed that both chronic restraint and α-adrenergic blockade by itself significantly increased metastasis (P values < 0.05; Fig. 2C–D).
Figure 2.
Effect of α-adrenergic blockade on breast cancer metastasis to distant tissues under non-stress and stress conditions. (A) Distant metastasis was measured over 28 days, *** P < 0.001 for group effect. (B) Representative images of distant metastatic sites. (C) Ex vivo analysis of lung metastases, a: P < .05 vs. Control, b: P < .05 vs. Restraint+Phentolamine. (D) Representative images of lungs showing tumor-specific bioluminescent signal. Y-axis: × 106 photons/sec in (A) and (C).
3.2. Direct effects of α-adrenergic blockade on breast cancer cell proliferation
To determine whether the effects of α-adrenergic blockade in vivo were the result of direct signaling to tumor cells, we investigated the effect of drug signaling on tumor cell proliferation in vitro in the absence and presence of catecholamines. The effect of phentolamine on cell proliferation was not significantly different from untreated cells (P = 0.739; Suppl. Fig. 1A). Also, the combination of either epinephrine or norepinephrine with an equivalent concentration of phentolamine had no significant effect on cell proliferation (P values > 0.854; Suppl. Fig. 1B–C). These in vitro results suggested that the effects of α-adrenergic blockade in vivo under non-stress and stress conditions were likely due to indirect mechanisms.
3.3. Activation of the sympatho-adrenomedullary and sympatho-neural systems
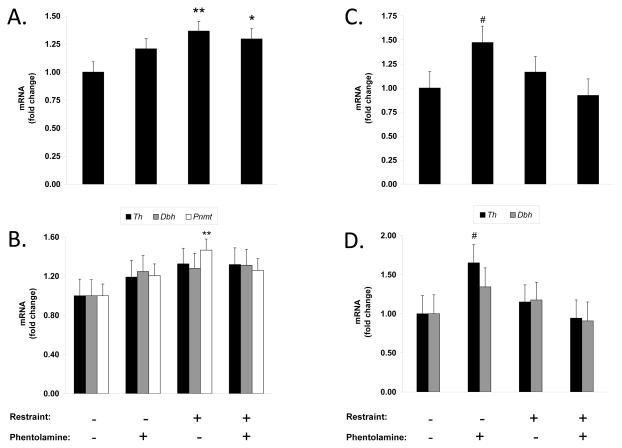
To investigate the possibility that the effects of α-blockade under non-stress and stress conditions resulted from differential activity levels in catecholaminergic systems, we measured gene expression of the key biosynthetic enzymes for peripheral catecholamine production in the sympatho-adrenomedullary and sympatho-neural systems. Tyrosine hydroxylase (Th) is the rate-limiting enzyme that initiates catecholamine synthesis; dopamine β-hyrdoxylase (Dbh) synthesizes norepinephrine; phenylethanolamine N-methyltransferase (Pnmt) converts norepinephrine to epinephrine. Chronic exposure to a variety of stressors increases expression of mRNA for Th, Dbh, and Pnmt in the adrenal medulla and Th and Dbh in sympathetic ganglia as a compensatory response to extensive catecholamine release during repeated stressful episodes (Kvetnansky et al., 2009). Chronic restraint significantly increased adrenal gland catecholaminergic gene expression (composite of Th, Dbh, and Pnmt) by 37% vs. controls (P = 0.005, Fig. 3A). Although each of the component genes were increased by chronic restraint, Pnmt elevation appeared to be the most sensitive (Th, 32%, Dbh, 28%, Pnmt, 47%, Fig. 3B). The effect of chronic restraint on catecholaminergic gene expression was not modified by α-adrenergic blockade (30% vs. controls, Fig. 3A; Th, 32%, Dbh, 31%, Pnmt, 26%, Fig. 3B), suggesting that the inhibitory effect of α-blockade on stress-enhanced cancer progression was mediated independently of adrenal gland-catecholamine activation. α-adrenergic blockade under non-stress conditions tended to increase adrenal catecholaminergic gene expression, but mean level did not reach significance (P = 0.112). We also measured mRNA expression of the catecholamine-degrading enzymes, monoamine oxidase A and B (Maoa, Maob). There was no significant effect of restraint on Maoa or Maob expression in the adrenal gland vs. controls (i.e., no restraint, no phentolamine) (P values > 0.485; Suppl. Fig. 2A–B). α-adrenergic blockade tended to increase adrenal gland Maoa expression in both the non-stress and stress groups vs. controls, but these levels did not reach significance (P values > 0.061; Suppl. Fig 2A). α-adrenergic blockade also had no significant effect on adrenal gland Maob expression in the non-stress and stress groups vs. controls (P values > 0.708; Suppl. Fig. 2B).
Figure 3.
Effect of α-adrenergic blockade on sympatho-adrenomedullary and sympatho-neural system activation under non-stress and stress conditions. (A) Composite gene expression of tyrosine hydroxylase (Th), dopamine β-hydroxylase (Dbh), and phenylethanolamine N-methyltransferase (Pnmt) in the adrenal. (B) Individual gene expression for Th, Dbh, and Pnmt in the adrenal. (C) Composite gene expression of Th and Dbh in thoracic sympathetic ganglia. (D) Individual gene expression for Th and Dbh in thoracic sympathetic ganglia. # P = .05, * P < .05, **P < .01 vs. respective control.
In sympathetic ganglia, a different pattern of catecholaminergic gene expression occurred (composite of Th and Dbh, Fig. 3C). Here, chronic restraint had no significant effect on catecholaminergic gene expression (P = 0.483), and α-adrenergic blockade under stress conditions did not significantly alter catecholaminergic gene expression (P = 0.752). However, α-adrenergic blockade under non-stress conditions increased catecholaminergic gene expression in sympathetic ganglia by 47% relative to controls (P = 0.054, Fig. 3C), suggesting that non-selective α-blockade may increase the release of sympatho-neural catecholamines that then act on β-adrenergic response pathways relevant to breast cancer progression. Individual gene expression for Th was more sensitive to non-selective α-blockade than Dbh (Th, 65%, Dbh, 34%, Fig. 3D). For gene expression of catecholamine-degrading enzymes in sympathetic ganglia, neither restraint by itself nor α-adrenergic blockade in either stress condition significantly affected Maoa or Maob expression (P values > 0.264; Suppl. Fig. 2C–D).
3.4. Effect of β-blockade on α-blockade-enhanced breast cancer progression
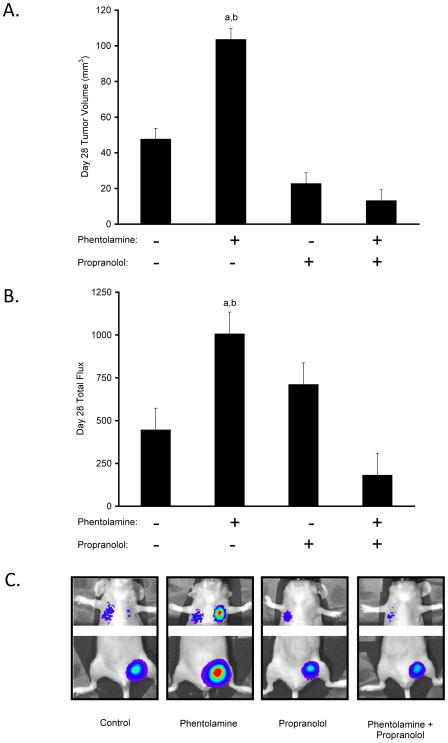
To further investigate the possibility that α-blockade mimicked chronic restraint enhancement of breast cancer progression by increasing sympatho-neural discharge and subsequent signaling of β-adrenergic receptors, we examined the effect of β-blockade on phentolamine-enhanced breast cancer progression. As shown in Figure 4, there was a significant phentolamine x propranolol interaction effect on primary tumor growth (P = 0.006) and metastasis (P = 0.021). Propranolol inhibited the effect of phentolamine on tumor volume, as mean primary tumor volume in the phentolamine plus propranolol group was significantly lower than in the phentolamine group (P = 0.001; Fig. 4A). Likewise, propranolol inhibited the effect of phentolamine on distant metastasis compared to the phentolamine group (P = 0.001; Fig. 4B–C).
Figure 4.
β-adrenergic signaling in α-blockade-enhanced breast cancer progression. (A) Group differences in primary tumor volume on Day 28. (B) Group differences in distant metastasis on Day 28. (C) Representative images of distant metastatic sites. a: P < .05 vs. Control, b: P < .05 vs. Phentolamine + Propranolol. Y-axis: × 103 photons/sec.
3.5. Effects of selective α1- and α2-adrenergic blockade on breast cancer progression under non-stress conditions
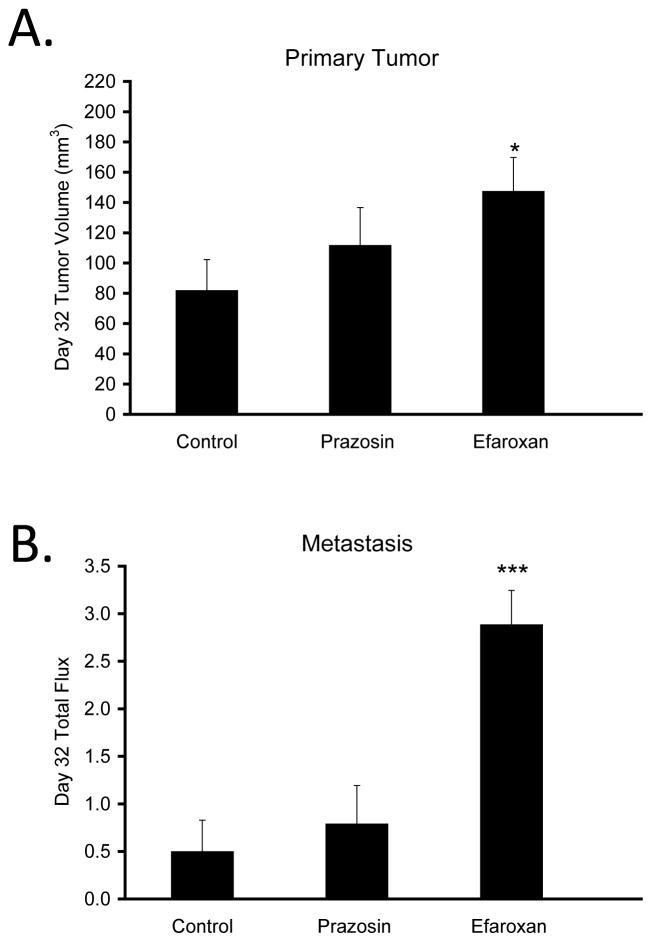
To determine whether the effects of α-blockade on breast cancer progression under non-stress conditions were mediated by a specific α-adrenergic receptor subtype, we examined the effects of selective α1- and α2-blockers. Under non-stress conditions, the α1-blocker, prazosin, had no significant effect on primary tumor volume (P = 0.358; Fig. 5A) or distant metastasis (P = 0.580; Fig. 5B) compared to controls. However, the α2-blocker, efaroxan, significantly increased both primary tumor volume (P < 0.001; Fig. 5A) and distant metastasis (P < 0.001; Fig. 5B).
Figure 5.
Effects of selective α1- and α2-adrenergic blockade on breast tumor growth and metastasis to distant tissues under non-stress conditions. Prazosin (α1-blocker) and efaroxan (α2-blocker) effects on (A) primary tumor volume and (B) distant metastasis on Day 32 under non-stress conditions. * P < 0.05, *** P < 0.001 vs. Control. Y-axis: × 106 photons/sec in (B).
4. Discussion
The α-adrenergic antagonist, phentolamine, inhibited the promoting effect of chronic restraint stress on both primary tumor growth and metastatic dissemination in a preclinical orthotopic model of human breast cancer. However, under non-stress conditions, phentolamine increased primary tumor size and distant metastasis at a rate that was commensurate with the effect of chronic restraint. We found no evidence that phentolamine’s effects in vivo stemmed from direct effects on MDA-MB-231HM breast cancer cell proliferation. However, phentolamine caused a higher level of catecholaminergic gene expression in the peripheral sympatho-neural system compared to other groups. Moreover, the non-selective β-blocker propranolol inhibited the enhancing effects of phentolamine on breast cancer progression. We further found that the enhancing effects of non-selective α-adrenergic antagonism on breast cancer progression were replicated with α2-blockade but not α1-blockade. These results suggest that α-adrenergic signaling may play a significant role in breast cancer progression in vivo, but they also underscore the challenge that may lie in attempts to therapeutically interrupt that signaling dynamic with α-adrenergic antagonism.
The present finding that α2-adrenergic blockade can mimic the enhancing effect of chronic stress on breast cancer progression is consistent with previous research in breast cancer models that indicate an increase in sympathetic activity can promote breast cancer progression by signaling through β-adrenergic receptors in the tumor microenvironment, which in turn can increase accumulation of tumor-associated macrophages, tumor angiogenesis, and the expression of metastasis-promoting genes (Thaker et al., 2006; Sloan et al., 2010). Several reports have shown that α2-adrenergic receptors mediate an autoinhibition of sympathetic transmission (Hein et al., 1999; Scheibner et al., 2001; Trendelenburg et al., 2001) and that disruption of this negative feedback signal with α2-adrenergic antagonists or gene inactivation of α2-adrenergic receptors enhances norepinephrine release (Starke, 2001). Subsequently, phentolamine, with affinity for both α1- and α2-adrenergic receptors, has been shown to increase norepinephrine release from peripheral tissues ex vivo (Schelb et al., 2001). Consistent with that reported effect on norepinephrine by phentolamine, we found that phentolamine increased catecholaminergic gene expression in the peripheral sympatho-neural system and that phentolamine’s effects on breast cancer progression were mediated through β-adrenergic receptors. Moreover, we confirmed phentolamine’s α2-adrenergic effect by showing that the selective α2-antagonist, efaroxan, also significantly increased breast cancer progression but the selective α1-antagonist, prazosin, did not.
It is unclear why phentolamine’s α2-adrenergic antagonism did not increase sympatho-neural gene expression and breast cancer progression in mice that were also undergoing chronic restraint. Given that phentolamine is a reversible competitive antagonist (Dinsmore & Wyllie, 2008), it is possible that the drug exerted less total antagonism of pre-synaptic α2-adrenergic receptors because of increased competitive agonism by elevated systemic levels of catecholamine from the adrenal medulla in chronically restrained mice. However, further research is needed to define these mechanisms.
Several observational studies have shown favorable outcomes for breast cancer patients using pharmacotherapy to block sympathetic signaling through β-adrenergic receptors (Powe et al., 2010; Melhem-Bertrandt et al., 2011; Barron et al., 2011; Botteri et al., 2013). The initial findings from the current preclinical study suggest the possibility that α-blockers exerting any α2-antagonism may increase sympathetic signaling and promote breast cancer progression through the β-adrenergic pathway. However, the inhibitory effect of phentolamine on cancer progression under stressful conditions in this study suggest that there may exist some circumstances in which α-blockers have potential clinical utility in breast cancer. Ultimately, more investigation in additional models of breast cancer is needed to test the generalizability of the current results. Also, further investigation of indirect mechanisms in the tumor microenvironment that likely mediate these results will determine whether such downstream mechanisms are similar to the ones found in previous studies (e.g., tumor-associated macrophage accumulation) and will allow for elucidation of the intracellular signaling pathways by which catecholamines regulate these pro-tumor effects. Given their seemingly paradoxical potential for help or harm in the context of breast cancer and the fact that many women take these drugs for conditions that include hypertension (Spatz et al., 2013), lower-urinary-tract symptoms (Boyd et al., 2014), and anxiety symptoms (Raskind et al., 2013), we think careful investigation of α-blockers in this disease is further warranted.
Supplementary Material
Supplementary Figure 1. Effect of α-adrenergic blockade and catecholamines on breast cancer cell proliferation in vitro at 72 hours post-incubation. (A) Phentolamine. (B) Phentolamine + Epinephrine. (C) Phentolamine + Norepinephrine.
Supplementary Figure 2. Gene expression for monoamine oxidase A (Maoa) and monoamine oxidase B (Maob). (A) Maoa in the adrenal. (B) Maob in the adrenal. (C) Maoa in thoracic sympathetic ganglia. (D) Maob in thoracic sympathetic ganglia.
Highlights.
Chronic restraint stress increased primary breast tumor growth and distant metastasis as expected
Pan α- and α2-blockade, but not α1, also increased primary tumor growth and distant metastasis
α2-adrenergic antagonism’s effect was mediated through β-adrenergic signaling
Acknowledgments
We thank Waldemar Ladno and Darin Williams for their assistance at the UCLA Crump Preclinical Imaging Center and Sabatino Ventura for discussion of alpha-adrenergic biology.
Role of the Funding Source
This project was supported in part by NCI grant R01CA160890 and National Breast Cancer Foundation (Australia) grant ECR-11-11 to EKS, NCI grant P30CA016042 to the UCLA Jonsson Comprehensive Cancer Center, and the UCLA Cousins Center for Psychoneuroimmunology.
Footnotes
Contributors
DML contributed to the literature review, overall study design and execution, statistical analysis, construction of data figures, and composed the initial manuscript drafts. HYS, GSY, JCYM, and JMD contributed to study execution and composition of the methods in the manuscript. SWC contributed to the overall study design, literature review, statistical analysis, and development of the final manuscript. EKS contributed to the overall study design, literature review, statistical analysis, and development of the final manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29:2635–44. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, Zanelotti A, Adamoli L, Colleoni M, Viale G, Goldhirsch A, Gandini S. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013;140:567–75. doi: 10.1007/s10549-013-2654-3. [DOI] [PubMed] [Google Scholar]
- Boyd K, Hilas O. α-Adrenergic Blockers for the Treatment of Lower-Urinary-Tract Symptoms and Dysfunction in Women. Ann Pharmacother. 2014;48:711–22. doi: 10.1177/1060028014524174. [DOI] [PubMed] [Google Scholar]
- Bruzzone A, Piñero CP, Castillo LF, Sarappa MG, Rojas P, Lanari C, Lüthy IA. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol. 2008;155:494–504. doi: 10.1038/bjp.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone A, Vanzulli SI, Soldati R, Giulianelli S, Lanari C, Lüthy IA. Novel human breast cancer cell lines IBH-4, IBH-6, and IBH-7 growing in nude mice. J Cell Physiol. 2009;219:477–84. doi: 10.1002/jcp.21694. [DOI] [PubMed] [Google Scholar]
- Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di GH, Jin W, Ou ZL, Shen ZZ, Shao ZM. Identification of the functional role of AF1Q in the progression of breast cancer. Breast Cancer Res Treat. 2008;111:65–78. doi: 10.1007/s10549-007-9761-y. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Dinsmore WW, Wyllie MG. Vasoactive intestinal polypeptide/phentolamine for intracavernosal injection in erectile dysfunction. BJU Int. 2008;102:933–7. doi: 10.1111/j.1464-410X.2008.07764.x. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct a2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–84. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Ascher DB, McLeod VM, Herold MJ, Le CP, Sloan EK, Porter CJ. PEGylation of interferon α2 improves lymphatic exposure after subcutaneous and intravenous administration and improves antitumour efficacy against lymphatic breast cancer metastases. J Control Release. 2013;168:200–8. doi: 10.1016/j.jconrel.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- Madden KS, Szpunar MJ, Brown EB. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res Treat. 2011;130:747–58. doi: 10.1007/s10549-011-1348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni L, Di Fausto V, Fiore M, Aloe L. Repeated restraint and nerve growth factor administration in male and female mice: effect on sympathetic and cardiovascular mediators of the stress response. Curr Neurovasc Res. 2008;5:1–12. doi: 10.2174/156720208783565654. [DOI] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. 2. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–52. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Piñero C, Bruzzone A, Sarappa MG, Castillo LF, Lüthy IA. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br J Pharmacol. 2012;166:721–36. doi: 10.1111/j.1476-5381.2011.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–38. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J, Millard SP, Rohde K, O’Connell J, Pritzl D, Feiszli K, Petrie EC, Gross C, Mayer CL, Freed MC, Engel C, Peskind ER. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003–10. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Scheibner J, Trendelenburg AU, Hein L, Starke K. Frequency-noradrenaline release relationships examined in a2A-, a2B- and a2C-adrenoceptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:321–8. doi: 10.1007/s002100100432. [DOI] [PubMed] [Google Scholar]
- Schelb V, Göbel I, Khairallah L, Zhou H, Cox SL, Trendelenburg AU, Hein L, Starke K. Postnatal development of presynaptic receptors that modulate noradrenaline release in mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:359–71. doi: 10.1007/s002100100455. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Research. 2010;70:7042–52. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz ES, Canavan ME, Desai MM, Krumholz HM, Lindau ST. Sexual activity and function among middle-aged and older men and women with hypertension. J Hypertens. 2013;31:1096–105. doi: 10.1097/HJH.0b013e32835fdefa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Presynaptic autoreceptors in the third decade: focus on alpha2-adrenoceptors. J Neurochem. 2001;78:685–93. doi: 10.1046/j.1471-4159.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 2013;6:1262–72. doi: 10.1158/1940-6207.CAPR-13-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Klebroff W, Hein L, Starke K. A study of presynaptic alpha2-autoreceptors in alpha2A/D-, alpha2B- and alpha2C-adrenoceptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:117–30. doi: 10.1007/s002100100423. [DOI] [PubMed] [Google Scholar]
- Vázquez SM, Pignataro O, Luthy IA. Alpha2-adrenergic effect on human breast cancer MCF-7 cells. Breast Cancer Res Treat. 1999;55:41–9. doi: 10.1023/a:1006196308001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Effect of α-adrenergic blockade and catecholamines on breast cancer cell proliferation in vitro at 72 hours post-incubation. (A) Phentolamine. (B) Phentolamine + Epinephrine. (C) Phentolamine + Norepinephrine.
Supplementary Figure 2. Gene expression for monoamine oxidase A (Maoa) and monoamine oxidase B (Maob). (A) Maoa in the adrenal. (B) Maob in the adrenal. (C) Maoa in thoracic sympathetic ganglia. (D) Maob in thoracic sympathetic ganglia.