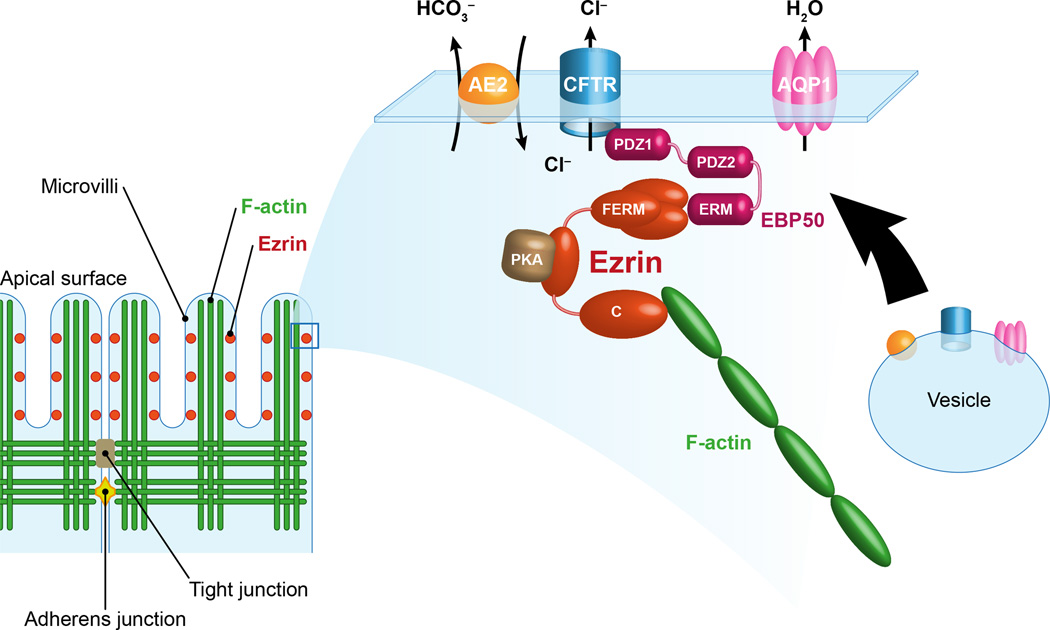
Figure 1. Proposed model for the regulation of bile secretion by ezrin in cholangiocytes.
Ezrin is localized at the apical actin-rich region of cholangiocytes where it contributes to the organization of multiprotein complexes. Ezrin regulates the membrane localization and the activation of CFTR. The FERM domain of ezrin anchors CFTR indirectly through the PDZ protein EBP50, whereas the C-term domain interacts with F-actin promoting the stabilization of the channel at the membrane. Ezrin can bind additional proteins such as PKA, thereby contributing to the regulation of CFTR. By acting as a protein kinase A anchoring protein (AKAP), ezrin positions the PKA near CFTR to be phosphorylated and activated. Furthermore, the activation of CFTR is spatially and functionally coordinated with the activation of AE2 and AQP1. This secretory complex already co-localizes in intracellular vesicles whose trafficking and membrane insertion are regulated through the interaction with actin cytoskeleton and by PKA activation. The involvement of ezrin in the insertion/stabilization and regulation of AE2 and AQP1 is currently undetermined. Thus, the architecture of the subcortical cytoskeleton and the distribution and retention of proteins at the apical membrane are important signals to maintain the apical polarity and the secretory functions of the epithelium.