Abstract
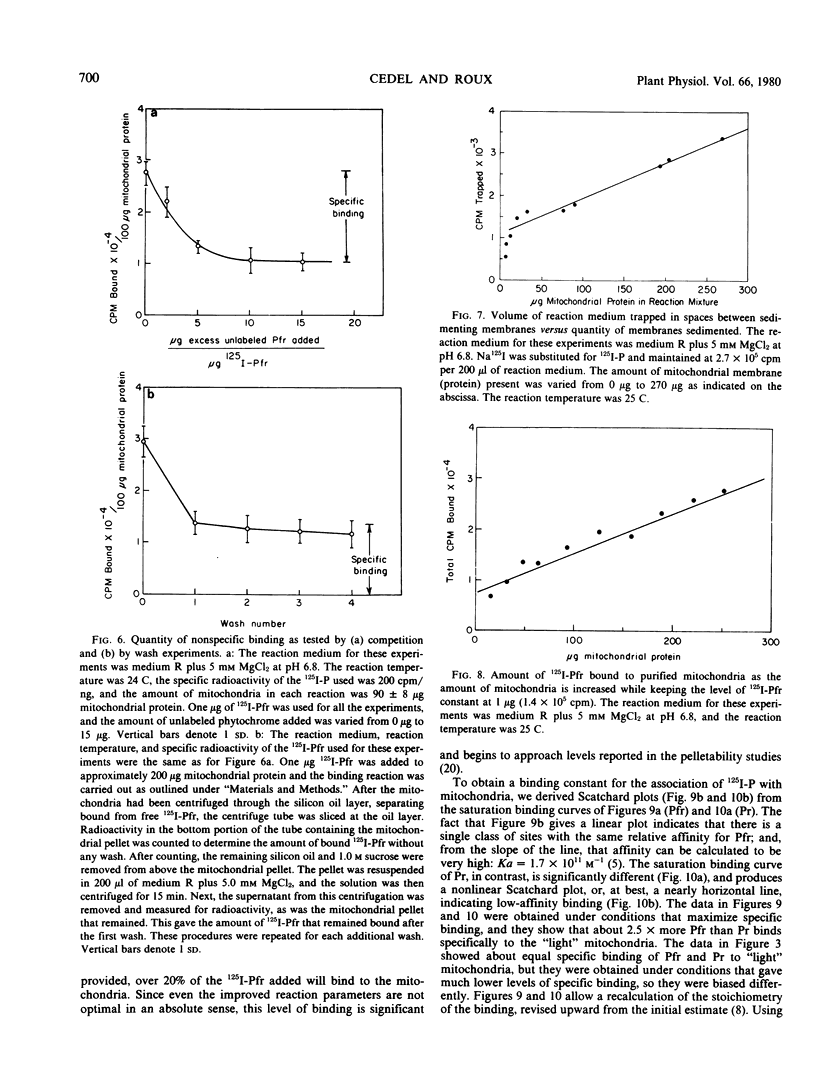
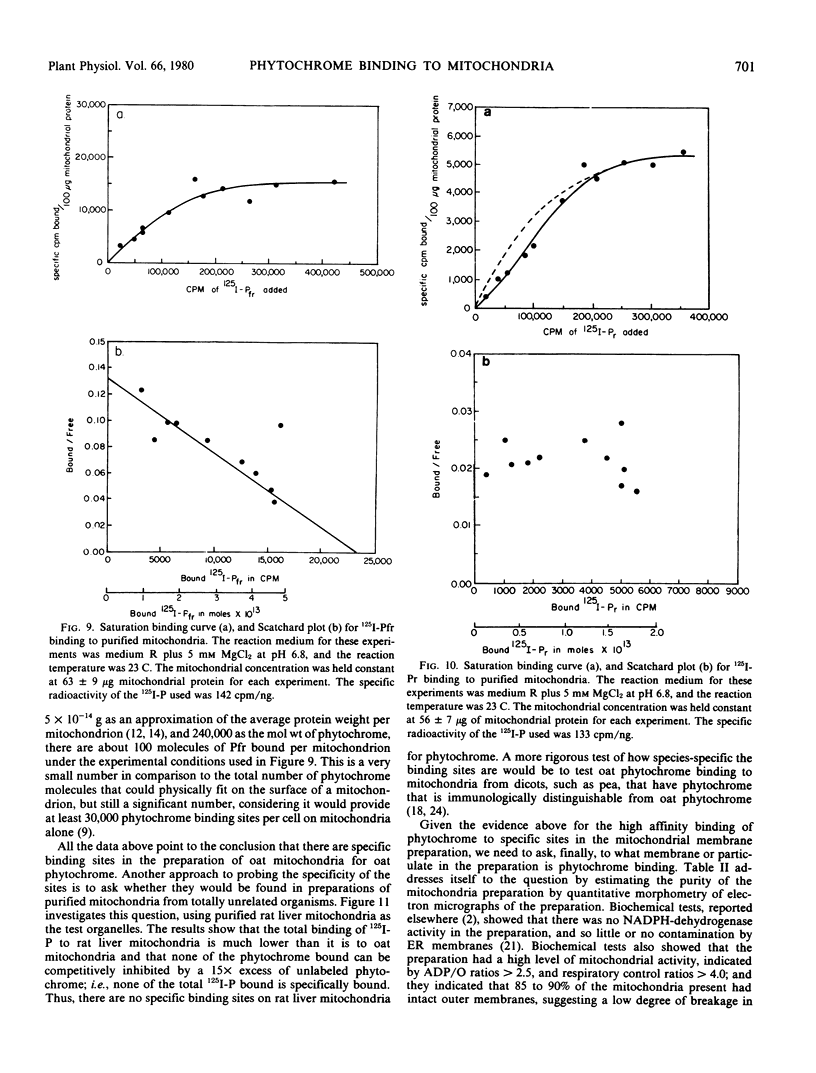
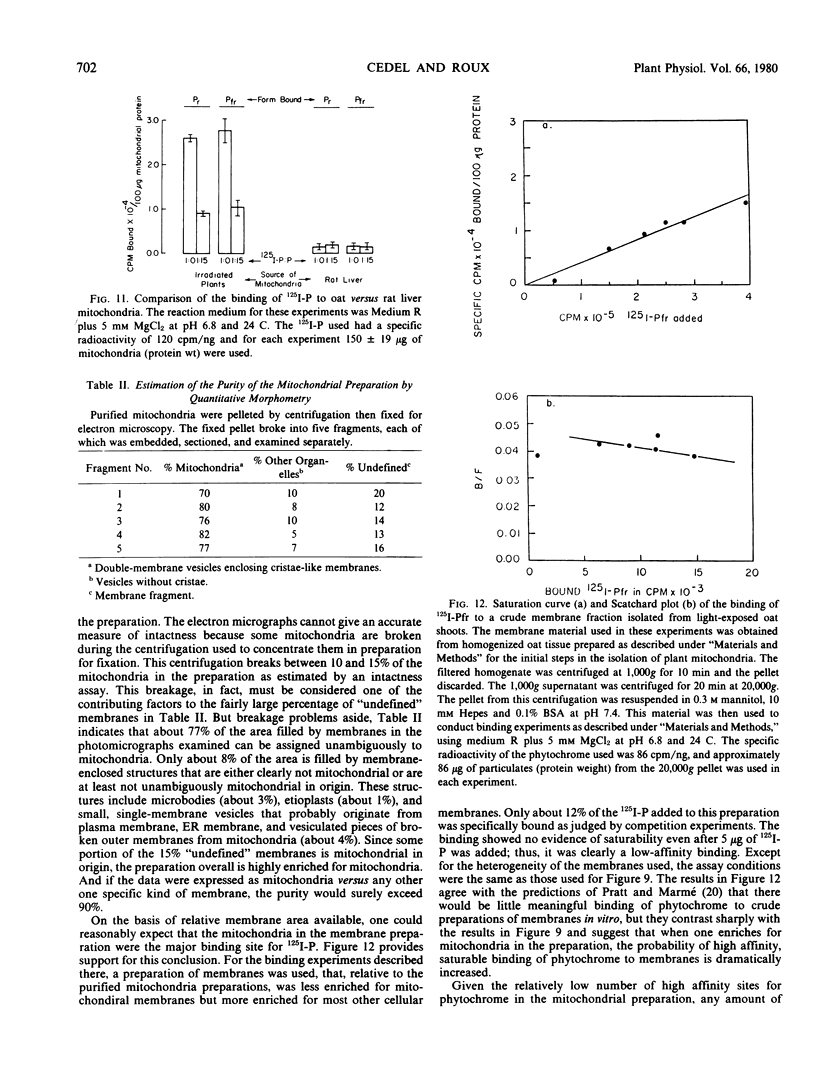
This study employs 125I-labeled phytochrome (125I-P) from oats to quantitate the binding of phytochrome to a membrane fraction from oats that is highly enriched for mitochondria, and it examines several parameters that influence this attachment. The binding of 125I-Pfr to the mitochondrial fraction of unirradiated oat seedlings is significantly higher than that of 125I-Pr. However, 125I-Pfr and 125I-Pr bind in equal quantities to mitochondrial preparations isolated from light-exposed seedlings. Maximum 125I-Pfr binding to membranes from light-exposed plants occurs within 30 seconds and is optimized in a reaction buffer containing 5 millimolar MgCl2 at pH 6.8. Scatchard plots of the binding data for Pfr indicate a single high-affinity site with an affinity constant of 1.79 × 1011 per molar. When optimal binding conditions are used, over 20% of the 125I-P added is bound and a stoichiometry of about 100 molecules per mitochondrion is attained. When the specificity of binding is tested using competition experiments with a 15-fold excess of unlabeled phytochrome, 125I-Pfr shows no specific binding to rat liver mitochondria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudhuin P. Morphometry of subcellular fractions. Methods Enzymol. 1974;32:3–20. doi: 10.1016/0076-6879(74)32004-6. [DOI] [PubMed] [Google Scholar]
- Cedel T. E. Modulation of a mitochondrial function by oat phytochrome in vitro. Plant Physiol. 1980 Oct;66(4):704–709. doi: 10.1104/pp.66.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Dahlquist F. W. The meaning of Scatchard and Hill plots. Methods Enzymol. 1978;48:270–299. doi: 10.1016/s0076-6879(78)48015-2. [DOI] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Georgevich G., Cedel T. E., Roux S. J. Use of I-labeled phytochrome to quantitate phytochrome binding to membranes of Avena sativa. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4439–4443. doi: 10.1073/pnas.74.10.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Newman I. A., Briggs W. R. Phytochrome-mediated Electric Potential Changes in Oat Seedlings. Plant Physiol. 1972 Dec;50(6):687–693. doi: 10.1104/pp.50.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Marmé D. Red Light-enhanced Phytochrome Pelletability: Re-examination and Further Characterization. Plant Physiol. 1976 Nov;58(5):686–692. doi: 10.1104/pp.58.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H., Briggs W. R. Irradiation-enhanced Phytochrome Pelletability: Requirement for Phosphorylative Energy in Vivo. Plant Physiol. 1978 Nov;62(5):773–778. doi: 10.1104/pp.62.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Immunochemistry of phytochrome. Plant Physiol. 1973 May;51(5):939–945. doi: 10.1104/pp.51.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]