FIG 3.
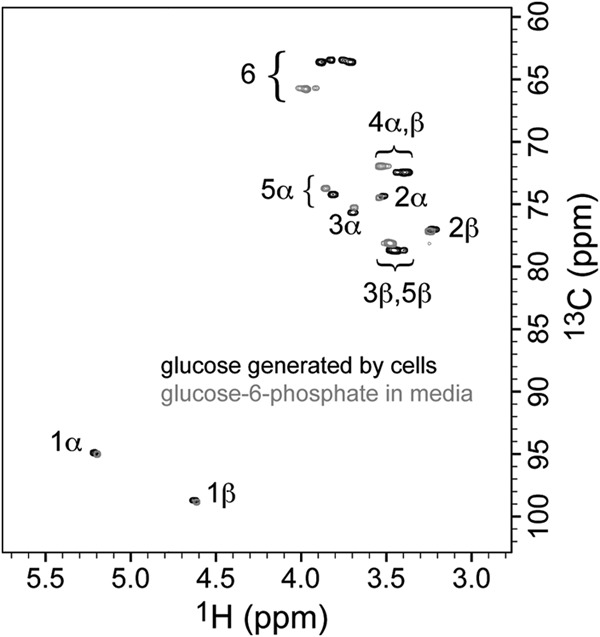
Confirmation of d-glucose production from xylose, using an overlay of the two-dimensional, 1H,13C-HSQC NMR spectra of a sample of product from the pentose (black contours, medium after xylose was exhausted by cells, with D2O added to 7%) and a sample using authentic glucose-6P in medium (gray contours, with D2O added to 7%). The molecules are distinguished because the presence of the phosphate group on d-glucose-6P promotes characteristic 1H and 13C chemical shift changes, compared to d-glucose, for the nuclei at positions nearer the site of glucose attachment (positions 6, 5, and 4, with smaller changes at 3, 2, and 1). Comparison with spectra of authentic d-glucose (not shown) confirms the identities. The data demonstrate that d-glucose is the fermentation product from xylose and that d-glucose does not form by extracellular hydrolysis of d-glucose-6P under the conditions of the experiments.
