Figure 2.
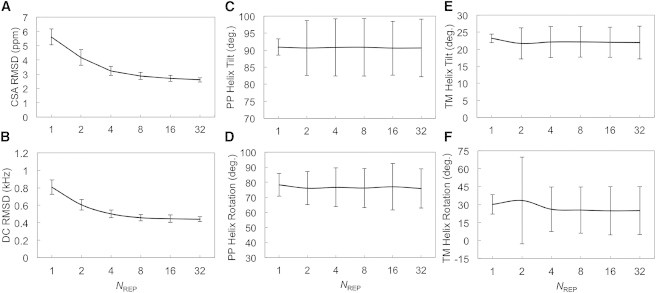
Validation of fd coat protein structure ensemble. (A and B) CSA and DC RMSD with respect to the experimental observables as a function of the number of replicas per ensemble simulation. (C and D) Tilt and rotation angles of the periplasmic helix of fd coat protein. (E and F) Tilt and rotation angles of the transmembrane helix of fd coat protein. The tilt angle is defined as the angle between the helix principal axis and the lipid bilayer normal. The rotation angle is defined as the angle between the perpendicular vector (rs) from the helical axis to a Cα atom (S13 for the periplasmic helix and G34 for the transmembrane helix) and the projection vector (zp) of the Z axis onto the plane made by the second and third principal axes. The sign of the rotational angle becomes positive if zp × rs is in the opposite direction to the helical axis or negative otherwise. The error bars are the standard deviations from the average.
