Figure 2.
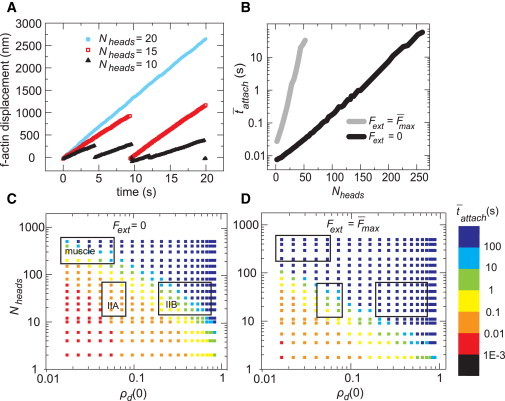
Myosin filament processivity depends on motor properties and external force. (A) Simulated F-actin trajectories for different values of Nheads. The actin filament was assumed to return to its original position upon release by the myosin. Each curve represents a single simulation. (B) The mean attached time as a function of Nheads showed a shift under stalled compared with unloaded conditions. (C) Mean attached time on an unloaded F-actin for a range of Nheads and ρd(0). The boxes indicate published values for muscle myosins, nonmuscle myosin IIB, and nonmuscle myosin IIA (see main text for references) starting at the top left and going clockwise. (D) Increased mean attached time for a stalled F-actin over the same range as in (C). Parameter values: (A) ρd(0) = 0.34 (kon = 10s−1, koff(0) = 19.1 s−1), Fext = 4 pN; (B) ρd(0) = 0.05 (kon = 10 s−1 and koff(0) = 191 s−1); (C and D) kon = 10 s−1. Averages in (B)–(D) were taken over 1000 s of simulation time. The standard error in B was smaller than the data points. The distribution of tattach is described in Fig. S9. To see this figure in color, go online.
