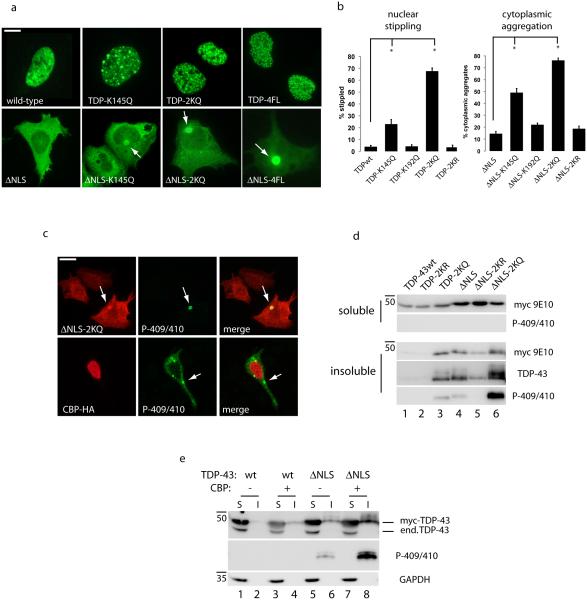
Figure 2. TDP-43 acetylation promotes pathological features associated with TDP-43 proteinopathies.
a) Double-labeling immunofluorescence was performed on cells transfected with myc-tagged wild-type TDP-43 or cytoplasmic localized TDP-43-ΔNLS containing the single acetylation mimic (K145Q), double mimic (TDP-2KQ), or 4FL RNA-binding deficient mutations (F147/149/229/231L). White arrows highlight cytoplasmic accumulation of TDP-43 aggregates. Scale bar represents 10 µm. b) Nuclear stippling and cytoplasmic aggregation phenotypes were quantified for all TDP-43 acetylation-mimic and non-mimic mutants at residues Lys-145 and Lys-192. Error bars indicate standard error of the mean (SEM), and the single asterisk indicates statistical significance with p-value < .05 as measured by two-tailed unpaired t-test with unequal variance from N=3 biological replicates. c) Cells expressing acetylation-mimic TDP-43-ΔNLS (TDP-43-ΔNLS-2KQ) or CBP-acetylated TDP-43-ΔNLS were analyzed by immunofluorescence microscopy using a phospho-TDP-43 antibody (P-409/410) to mark pathological TDP-43 aggregates. Scale bar represents 25 µm. d-e) Soluble (S) and insoluble (I) fractions from cells expressing acetylation-mimics as well as CBP-mediated acetylated TDP-43 were analyzed by immunoblotting using the indicated myc-9E10, TDP-43 (1038), phospho-TDP-43 (P409/410), or GAPDH antibodies.