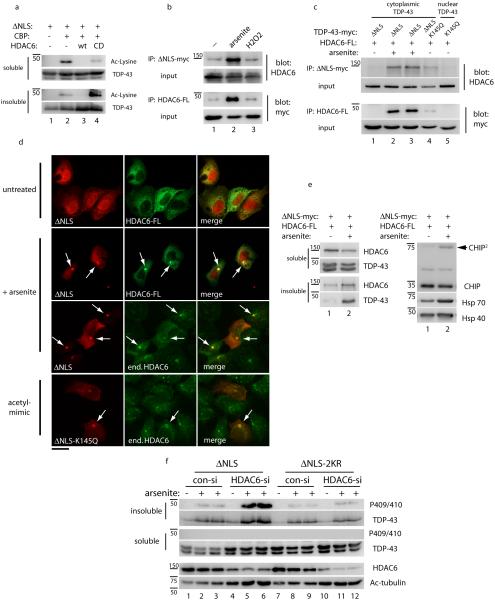
Figure 5. The deacetylase HDAC6 binds and deacetylates TDP-43 in a stress-regulated manner.
a) Cells were co-transfected with TDP-43-ΔNLS, CBP, and either wild-type HDAC6 or the catalytically dead HDAC6-H611A mutant and TDP-43 acetylation was determined by immunoblotting with acetyl-lysine antibodies similar to Figure 1c. b) Cells transfected with myc-tagged TDP-43-ΔNLS and FLAG-tagged HDAC6 were analyzed by co-immunoprecipitation reactions under basal or stimulated conditions in the presence of 0.1 mM arsenite or 1 mM peroxide stress for 2 hr. Input, representing 5% of total lysate volume, was analyzed by immunoblotting using the indicated antibodies. c) Co-immunoprecipitation analysis using either TDP-43-ΔNLS or acetylation mimic mutants (TDP-43-K145Q or TDP-43-ΔNLS-K145Q) and wild-type HDAC6 were performed in the absence or presence of arsenite, and immunoprecipitated proteins were analyzed by western blotting with the indicated antibodies similar to b above. d) Immunofluorescence microscopy was performed on cells expressing myc-tagged TDP-43-ΔNLS, acetylation mimic TDP-43-ΔNLS-K145Q, and HDAC6-FLAG where indicated, using anti-FLAG or endogenous HDAC6 antibodies. TDP-43 acetylation-mimic mutations or acute treatment with arsenite led to the formation of cytoplasmic aggregates that co-localized with HDAC6. The scale bar represents 25 µm. e) Protein levels of the HDAC6/CHIP/Hsp70 complex were determined by western analysis of soluble and insoluble fractions using HDAC6, CHIP, Hsp70, and Hsp40 specific antibodies. Note, CHIP dimers (arrowhead) suggest activation of CHIP E3 ligase function, as previously reported68. f) Depletion of HDAC6 was performed using a specific HDAC6 siRNA followed by arsenite treatment, and solubility of TDP-43-ΔNLS and TDP-43-ΔNLS-2KR proteins were analyzed by immunoblotting with total and phosphorylated TDP-43 antibodies. HDAC6 siRNA knock-down efficiency was validated by assessing total levels of HDAC6 and its major substrate acetylated tubulin.