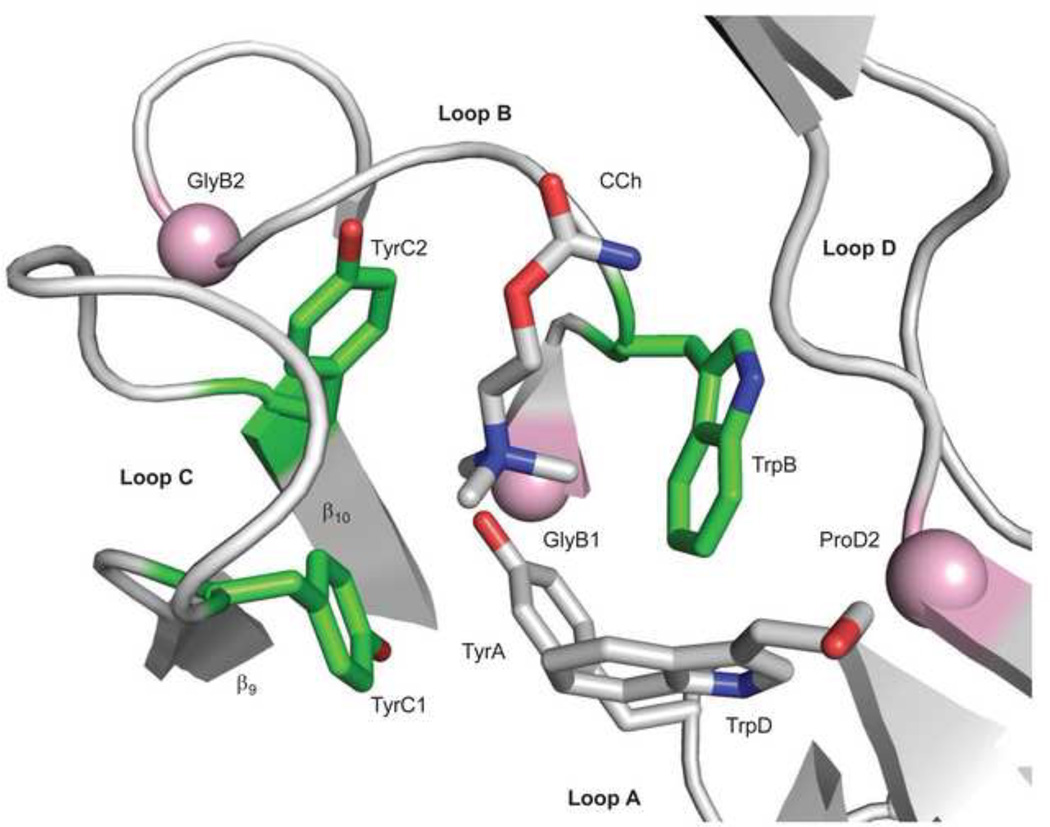
Figure 5. Sources of ΔGB.
The ligand binding site of AChBP with carbamylcholine (CCh)(pdb accession number 1uv6; 85). The view is from extracellular solution of a subunit interface. Left, the ‘principal’ subunit that corresponds to α in AChRs (loops A, B and C) and right, the ‘complimentary’ subunit that corresponds to ε/γ or δ in AChRs (loop D). An ‘aromatic triad’ (green) provides most of the ΔGBACh energy (~2 kcal/mol each, from the indole and benzene rings of TrpB, TyrC1 and TyrC2). The pink spheres are the Cα atoms of the residues that correspond to GlyB1, GlyB2 and ProD2 in AChRs. Mouse numbering: TyrA=αY93; GlyB1=αG147; TrpB=αW149; GlyB2=αG153; TyrC1=αY190; TyrC2=αY198; TrpD=εW55 or δW57; ProD2=εP121 or δP123.