FIGURE 1.
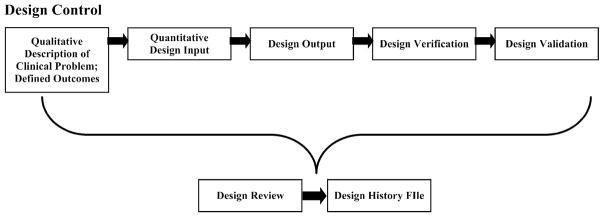
Schematic of the design control process. The process begins with definition of the specific clinical problem through quantitative design inputs. Design outputs are tests developed to characterize specific aspects of the engineered therapy. Design verification is the process of showing design outputs = design inputs, i.e., that the engineered therapy produced is equivalent to the one designed. Finally, design hypotheses concerning how the engineered system affects tissue response in bench, pre-clinical animal models and clinical trials are tested in design validation. The whole process is continually developed and assessed in design review, and all discussions and outcomes are documented in the Design History File (DHF).
