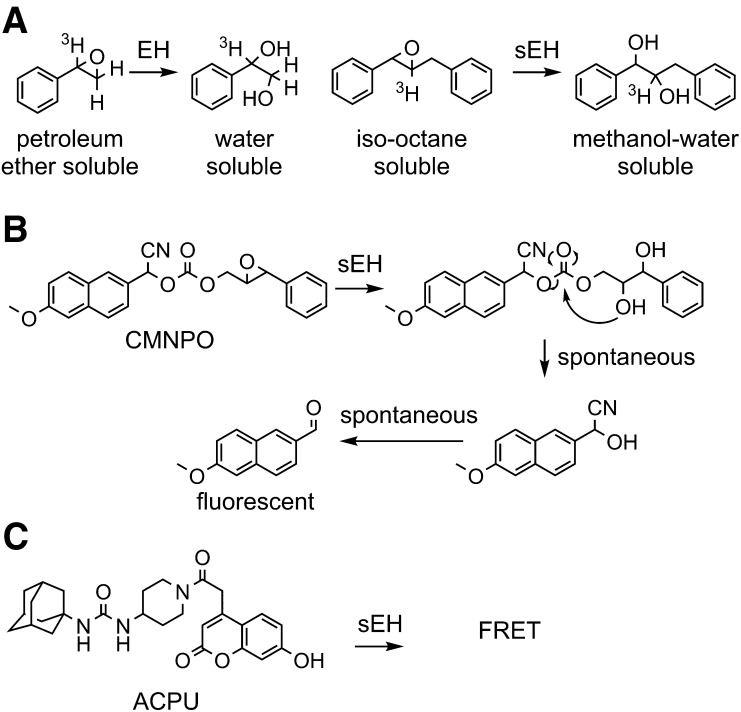
Fig. 4.
Methods for measuring epoxide hydrolase activity. Several substrates have been generated for rapid analysis of epoxide hydrolase enzymes and more sophisticated kinetic treatments. (A) The radioactive [7-3H] styrene oxide developed by Oesch et al. (1971a) used water solubility of the diol to monitor the reaction kinetics, and a similar partition method was used for trans-β-ethyl styrene oxide (Table 2) (Mullin and Hammock, 1980), trans-stilbene oxide (Table 2) (Gill et al., 1983), and trans-diphenylpropene oxide (Borhan et al., 1995). (B) More recent substrates rely on cyclization of the epoxide hydration product to release a chromophore. In the case of CMNPO, the prochromophore is a nonfluorescent cyanohydrin that spontaneously hydrolyzes to yield CN−, for which there are sensitive reagents, and an intensely fluorescent, red-shifted methoxynapthaldehyde (Jones et al., 2005). (C) There are a variety of techniques for high-throughput analysis of binding of a fluorescent molecule, such as ACPU designed for the sEH. In this case, it was used to determine a Ki value for the enzyme and a kinetic off rate to give an estimation of substrate occupancy of the catalytic site (Lee et al., 2013). ACPU, 1-(adamantan-1-yl)-3-(1-(2-(7-hydroxy-2-oxo-2H-chromen-4-yl)acetyl)piperidin-4-yl)urea; CMNPO, cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl] carbonate; EH, epoxide hydrolase; FRET, Förster resonance energy transfer.