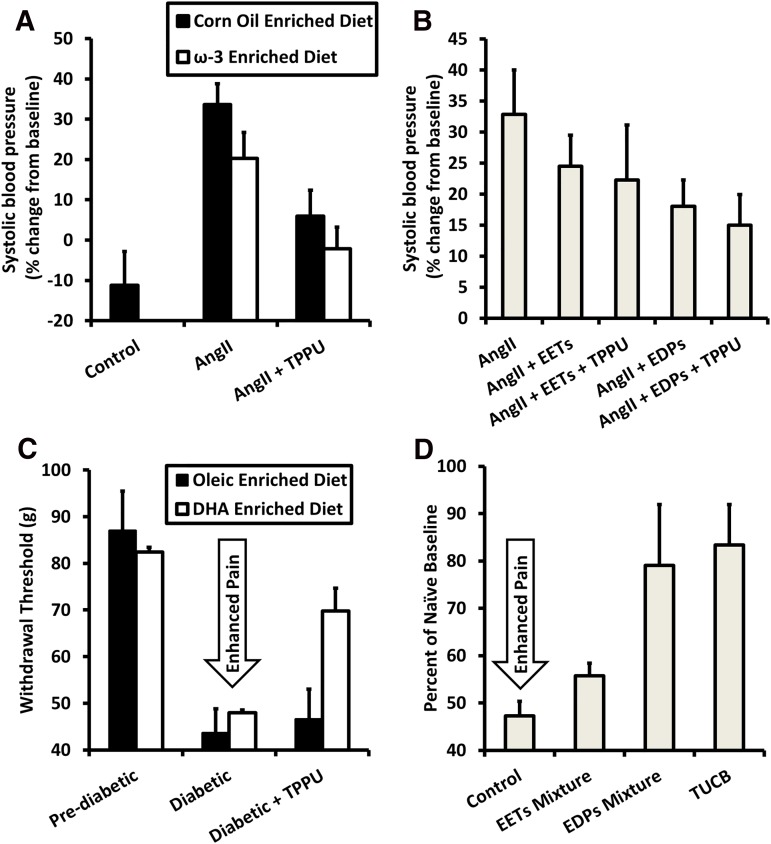
Fig. 7.
Efficacy of sEH inhibitors may be improved by supplementing ω-3 fatty acids in the diet by producing EpDPEs. (A) Either sEH inhibition or an ω-3–rich diet alone partially reduce increase in blood pressure 7 days after AngII injection, while giving both simultaneously reduces blood pressure close to control levels. Data are replotted from Ulu et al. (2013). (B) EpDPEs are likely the metabolites responsible for the improved efficacy of simultaneous administration of the ω-3 diet and sEH inhibitors. Slow release of EpDPEs into the bloodstream achieved by surgical implantation of osmotic minipumps resulted in decreased blood pressure 6 days after AngII injection and increasing half-life of EpDPEs by inhibiting sEH increased efficacy. By contrast, EETs with sEH inhibitors were not as potent toward reducing blood pressure. Data are replotted from Ulu et al. (2014). (C) In a rat model of streptozocin-induced diabetic neuropathy, an ω-3–rich diet had minimal effect at increasing pain thresholds relative to those on an oleic oil–based diet. However, when administering subtherapeutic levels (0.3 mg/kg) of sEH inhibitors, rats fed ω-3–rich food had an increased threshold relative to those on a control diet. Data are replotted from Wagner et al. (2014a). (D) As seen in hypertension, EpDPEs are likely responsible for the improved efficacy when both an ω-3 diet and sEH inhibitors are used to treat diabetic neuropathy. EpDPEs administered by intraperitoneal injection (1 mg/kg) in a mouse model of diabetic neuropathy were more efficacious at reducing pain than EETs and had a response similar to the sEH inhibitor t-TUCB (10 mg/kg). Data are replotted from Wagner et al. (2014a). DHA, docosahexaenoic acid; EDP, Epoxydocosapentaenoic Acid; TPPU, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea; t-TUCB, trans-4-{4-[3-(4-trifluoromethyoxy-phenyl)-ureido]-cyclohexyloxy}benzoic acid.