Abstract
Prenatal exposure to diethylstilbestrol (DES) is known to cause an increased susceptibility to a wide array of clinical disorders in humans. Previous studies from our laboratory demonstrated that prenatal exposure to DES induces thymic atrophy and apoptosis in the thymus. In the current study, we investigated if such effects on the thymus result from alterations in the expression of microRNA (miR). To that end, pregnant C57BL/6 mice who were exposed to DES and miR profiles in thymocytes of both the mother and fetuses on postnatal day 3 (gestation day 17) were studied. Of the 609 mouse miRs examined, we noted 59 altered miRs that were common for both mothers and fetuses, whereas 107 altered miRs were specific to mothers only and 101 altered miRs were specific to fetuses only. Upon further analyses in the fetuses, we observed that DES-mediated changes in miR expression may regulate genes involved in important functions, such as apoptosis, autophagy, toxicity, and cancer. Of the miRs that showed decreased expression following DES treatment, miR-18b and miR-23a were found to possess complementary sequences and binding affinity for 3′ untranslated regions of the Fas ligand (FasL) and Fas, respectively. Transfection studies confirmed that DES-mediated downregulation of miR-18b and miR-23a led to increased FasL and Fas expression. These data demonstrated that prenatal DES exposure can cause alterations in miRs, leading to changes in the gene expression, specifically, miR-mediated increased expression in FasL and Fas causing apoptosis and thymic atrophy.
Introduction
Diethylstilbestrol (DES) [4,4′-(3E)-hex-3-ene-3,4-diyldiphenol], a synthetic estrogen, was used in the United States (from the early 1940s to the 1970s) to prevent spontaneous abortion (Herbst, 1981; Laitman, 2002). Approximately 5–10 million expectant mothers and developing fetuses were exposed to DES during this period. DES exposure of mothers and fetuses during this time has caused long-term adverse effects. For example, mothers were shown to exhibit an increased risk of breast cancer (Herbst, 1981; Goodman et al., 2011), while DES-exposed daughters showed an increased risk of cervicovaginal cancer (Palmer et al., 2005; Hoover et al., 2011). Other abnormalities, such as immune system disorders, psychosexual effects, and reproductive abnormalities in DES daughters and sons, have also been reported (Giusti et al., 1995). There are studies that show prenatal exposure to DES alters immune functions in T cells as well as in other immune cells (Ford et al., 1983; Ways et al., 1987). An increased incidence of autoimmune diseases in adult life following prenatal DES exposure has also been reported (Noller et al., 1988). All these studies suggest that the immunologic effects of DES exposure may be far reaching in the offspring (sons and daughters) of exposed mothers.
DES exposure of prenatal mice has been shown to cause various abnormalities, including those in the thymus, skeletal tissues, female reproductive organs, and muscles (Takasugi, 1963; Maier et al., 1985; Okada et al., 2001). In the thymus, prenatal exposure to DES has been shown to cause thymic atrophy and several other changes, such as apoptosis, T cell differentiation, immunotoxicity, and immunosuppression (Ford et al., 1983; Brown et al., 2006a). We have also demonstrated that exposure to DES during pregnancy caused thymic atrophy in mothers as well as in fetuses and newborn pups (Brown et al., 2006b). Furthermore, we have also shown that DES induced alteration in positive and negative selection of T cells in the thymus (Brown et al., 2006a). Holladay et al. have shown that DES exposure caused postnatal alterations in T cell and natural killer cell functions and increased the incidence of autoimmune diseases (Holladay et al., 1993). There are studies demonstrating the effect of DES on the expression profile of several genes in the thymus (Doherty et al., 2010; Geier et al., 2010). In a recent study, Frawley et al. have reported DES-induced alteration in the gene expression profile in thymic cells (Frawley et al., 2011).
In recent years, microRNAs (miRs) have been investigated extensively for their role in gene expression. miRs are highly conserved noncoding single-stranded small RNA molecules (17–27 nucleotides). They control gene expression by binding the 3′ untranslated region (UTR) of the target gene mRNA post-transcriptionally. The binding of miRs with the target UTR of mRNA results in the degradation of the target mRNA or inhibition of the translation of the mRNA (Bartel, 2004; Iorio and Croce, 2009). miRs account for 1% of the genome, regulate 30% of the genome, and play a critical role in cellular processes, such as apoptosis, proliferation, and differentiation (Lee et al., 1993; Iorio and Croce, 2009).
While DES-induced alterations in gene expression have been well studied, the role of miRs in such a process remains unclear. In the current study, we therefore investigated if prenatal exposure to DES would alter miR expression in the thymus, an organ that has been well characterized to be the target of DES-induced toxicity. Our studies demonstrated specific alterations in miRs that alter the apoptotic pathways as well as other pathways, such as cancer. These studies demonstrate that DES-induced immunotoxicity may result from alterations in the expression of miR.
Materials and Methods
Mice.
Pregnant mice (C57BL/6) were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were kept in the University of South Carolina School of Medicine animal facility. The mice were cared for and maintained according to the guidelines for the care and use of laboratory animals as adopted by institutional and National Institutes of Health guidelines.
Cell Line.
EL4 cells were cultured in a complete medium (RPMI 1640 medium, 10% heat-inactivated fetal bovine serum, 10 mM l-glutamine, 10 mM HEPES, and 100 µg/ml penicillin/streptomycin) at 37°C and 5% CO2.
Chemicals.
We purchased DES [4,4′-(3E)-hex-3-ene-3,4-diyldiphenol] from Sigma-Aldrich (St. Louis, MO). DES suspended in dimethylsulfoxide was used in in vitro studies, and DES suspended in corn oil was used in in vivo studies. The following were reagents: RPMI 1640, HEPES, l-glutamine, penicillin/streptomycin and fetal bovine serum, Epicentre’s PCR premix F and platinum Taq polymerase kits, and a Lipofectamine RNAMAX transfection kit were purchased from Invitrogen Life Technologies (Carlsbad, CA). The miRNeasy kit, miScript cDNA synthesis kit, miScript primer assays kit, and miScript SYBR Green PCR kit were purchased from QIAGEN (Valencia, CA). EL4 transfection kits was purchased from Lonza Cologne GmbH (Cologne, Germany).
In Vivo Exposure of Pregnant Mice with DES.
We used a single dose of DES (5 μg/kg) in this study. DES was administered intraperitoneally into pregnant mice (C57BL/6) on gestation day (GD) 14 as described previously (Singh et al., 2012b). We performed dose-response studies and found that the dose of 5 μg/kg of the body weight was the optimal dose for our studies (Singh et al., 2012b). Administration of doses higher than this led to profound thymic atrophy, making it difficult to secure enough cells for the proposed experiments. This dose range has been used by others (Ohta et al., 2014). On day 3 (GD 17), thymi were harvested and thymic weight and cellularity were determined. Mice treated with vehicle (VEH) (corn oil) were used as control. For each treatment group, at least three pregnant mice were used, and from each pregnant mother, we obtained an average of 5–8 pups. We combined the three litters from each treatment group to generate a pool of 18–24 fetuses. Due to low thymic cellularity in the fetus, thymi from five fetuses were randomly pooled per sample and approximately five replicate pools were used for the statistical analysis.
Determination of Thymic Cellularity and Apoptosis Post-DES Exposure.
Thymi from mothers and fetuses were harvested and transferred in complete RPMI 1640 medium. We prepared single cell suspensions of thymi as described earlier (Camacho et al., 2004a, b). The cell viability was determined using an inverted phase contrast microscope. Thymic cellularity was expressed as the total number of thymocytes per mouse. We pooled 5–6 replicate pools, which were compared from each treatment group for statistical analysis, and data were depicted as mean ± S.E.M. Apoptosis in thymic cells post-DES exposure was determined as described previously (Singh et al., 2012b). Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assays were performed using a TUNEL kit from Roche Applied Sciences and following the protocol of the company (Indianapolis, IN). Apoptosis in thymocytes was analyzed using flow cytometry (CXP 500; Beckman Coulter, Brea, CA).
Determination of Fas and Fas Ligand Expression in Thymocytes.
Expression of Fas and Fas ligand (FasL) in the mother’s and fetal thymic cells was measured using reverse-transcription polymerase chain reaction (RT-PCR) as described earlier (Fisher et al., 2004). In brief, single cell suspension of thymic cells post-VEH or post-DES treatment was prepared as described above. Expression of Fas and FasL was determined by RT-PCR as described previously (Singh et al., 2008). For RT-PCR, total RNAs from VEH- or DES-treated groups were isolated using the RNeasy mini kit following the protocol of the company (QIAGEN, Germantown, MD). cDNA synthesis was performed in a 20 µl reaction mixture containing 1 µg total RNA using the iScript kit and following the protocol of the manufacturer (Bio-Rad, Hercules, CA). PCR was performed using mouse FasL- or Fas-specific sets of forward and reverse primers as described earlier (Singh et al., 2008). The PCR products, generated from mouse Fas and FasL primer pairs, were normalized against mouse-specific 18S PCR products as described previously (Singh et al., 2008). The intensity of PCR products was determined using the ChemiDoc image analysis system (Bio-Rad).
miR Array Assays.
Total RNAs, including miRs from the thymi of mothers and fetuses exposed to DES or VEH, were isolated using the miRNeasy kit from QIAGEN and following the manufacturer’s instructions. miR arrays were performed using the Affymetrix GeneChip miR platform (Santa Clara, CA). The data generated from miR arrays were analyzed using a hierarchical clustering and pathway network. Induction or repression of miR expression was determined using a two-sample t test as described previously (Singh et al., 2012a; Hegde et al., 2013), and a P value of <0.01 in the t test was considered significant. In this study, a fold change of more than 1.5-fold was considered positive.
Real-Time PCR to Validate the Expression of miRs in Thymocytes.
We selected several downregulated miRs (miR-18b, miR-23a, miR-30a, miR-31, miR-146a, miR-155, and miR-217) and one upregulated miR (miR-320) to validate their expression. Real-time PCR assays were performed on cDNAs generated from total RNAs, including miRs isolated from fetal thymocytes exposed to DES or VEH as described earlier. We used the miScript primer assays kit (details in Supplemental Table 1) and miScript SYBR green PCR kit from QIAGEN following the protocol of the company (QIAGEN).
We used the StepOnePlus real-time PCR system V2.1 (Applied Biosystems, Carlsbad, CA). The following conditions were used: 40 cycles for 15 minutes at 95°C (initial activation step), 15 seconds at 94°C (denaturing temperature), 30 seconds at 55°C (annealing temperature), and 30 seconds at 70°C (extension temperature and fluorescence data collection). The normalized expression of miRs was calculated, and the data were normalized to various miRs against the internal control miR (Snord96a). Fold change of miRs was calculated against control miR, and the DES treatment group was compared with the VEH group. Analysis of variance was performed using GraphPad version 4.0 (GraphPad Software, San Diego, CA) to define significant differences in miR levels in the thymocytes post-DES or post-VEH treated groups, and differences between treatment groups were considered significant when *P < 0.05.
Analysis of miRs and Their Association with Various Pathways.
Postgeneration of the heat map and analysis of miR expression, we selected dysregulated (upregulated/downregulated) miRs (>1.5 fold) in fetal thymi exposed to DES when compared with those exposed to VEH. Next, the selected miRs were analyzed for their role in the expression of various genes and pathways using IPA software and the database of the company (Ingenuity Inc., Redwood City, CA) as described previously (Singh et al., 2012a; Hegde et al., 2013). DES-regulated miRs were also analyzed using Cytoscape 3.0.1 and Cluego software (Cytoscape Consortium, San Diego, CA) as described previously (Singh et al., 2012a; Hegde et al., 2013).
miR-mRNA Target Interactions.
We identified miR-specific mRNA targets using microRNA.org (Memorial Sloan Kettering Cancer Center, New York, NY), TargetScan mouse 5.2 (Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology, Boston, MA), miRWalk (Heidelberg, Germany), and miRGEN (version 3; Diana Lab, Athens, Greece) software and their database as described earlier (Singh et al., 2012a; Hegde et al., 2013). The details of some of miRs and the UTR region of their target gene (RNA targets) are described in Supplemental Table 2.
Transfection with Mature miR-23a and miR-18b and Determination of Fas and FasL Expression in the Presence or Absence of DES.
EL4 cells (5 × 106) were transfected using the lipofectamine RNAMAX transfection kit from Invitrogen and following the protocol (reverse transfection) of the company (Life Technologies) as described previously (Singh et al., 2012a; Hegde et al., 2013). Forty-eight hours post-transfection, EL4 cells were treated with VEH or DES (10 μM/ml) for 24 hours, and the expression of Fas and FasL in EL4 cells was determined. To this end, total RNAs from EL4 cells transfected with miR-23a or miR-18b and treated with DES or VEH were isolated using the RNeasy mini kit from QIAGEN and following the protocol of the company. Fas and FasL expression was determined as described previously (Singh et al., 2012a). The PCR products generated from mouse Fas and FasL primer pairs were normalized against PCR products generated from mouse 18S after electrophoresis. The band intensity of PCR products was determined using the ChemiDoc image analysis system (Bio-Rad).
Generation of the Reporter Constructs Containing Fas UTR Region or FasL UTR Region.
To this end, we used the pmirGLO reporter vector from Promega Corporation (Madison, WI). The mouse-specific Fas UTR region or FasL UTR region was cloned into the pmirGLO vector, and these were designated as pmirGLO–Fas UTR or pmirGLO–FasL UTR as described previously (Singh et al., 2012a).
Generation of miR-23a–Specific Fas UTR or miR-18b–Specific FasL UTR Oligonucleotides.
First, oligonucleotide pairs containing SacI and XbaI restriction sites (forward and reverse) of the mouse Fas UTR region containing miR-23a–specific binding sites and the mouse FasL UTR region containing miR-18b–specific binding sites were generated by Integrated Device Technology (San Jose, CA). The details of the oligonucleotide pairs for the miR-23a–specific Fas UTR region and miR-18b–specific FasL UTR region are described below.
Oligonucleotide pairs (Fas UTR) of miR-23a:
Forward primer: 5′-AGTCGAGCTCGTTCCAGGGACTGCTTCTG-3′
Reverse primer: 5′-AGTCTCTAGAGGAAGGTCTTCAATTAACTGCGAG-3′
Oligonucleotide pairs (FasL UTR) of miR-18b:
Forward primer: 5′-AGTCGAGCTCAGCTTTGGGCTGCTGTGTG-3′
Reverse primer: 5′-AGTCTCTAGATGGTGCCAATGAGACACTGTC-3′
In the presence of the oligoannealing buffer (46 µl), 2 µl of each oligonucleotide of mouse Fas UTR (specific to miR-23a) or mouse FasL UTR (specific to miR-18b) was annealed at 90°C for 3 minutes and 37°C for 15 minutes. The annealed oligonucleotides of Fas UTR or FasL UTR were used immediately or stored at −20°C.
Ligation and Transformation.
Annealed oligonucleotides of Fas UTR or FasL UTR were ligated to pmirGLO vector restricted with SacI and XbaI following the protocol of the company (Promega Corporation). Ligated pmirGLO–Fas UTR or pmirGLO–FasL UTR was transformed, and positive clones were selected for further use after confirming the clones by sequencing.
Transfection of EL4 Cells and Luciferase Assays.
Freshly cultured EL4 cells (5 × 106) were transfected with 5–10 µg of the pmiRGLO plasmid without an insert (MOCK), pmirGLO–Fas UTR plasmid, pmirGLO–Fas UTR plasmid and mature miR-23a, pmirGLO–FasL UTR plasmid, or pmirGLO–FasL UTR plasmid and mature miR-18b using an Amaxa Nucleoector instrument and EL4 transfection kits from Lonza Cologne GmbH as described previously (Singh et al., 2012a). EL4 cells were replated in triplicate in a 96-well plate 2 days post-transfection, and the cells were treated with VEH or DES (10 µM/ml). The cells were incubated for 24 hours at 37°C, 5% CO2 post-treatments. The cells were harvested, washed twice with phosphate-buffered saline, and centrifuged, and extracts were prepared by lysing cells in 70 µl of reporter lysis buffer (Promega Corporation). Luciferase activity was determined using the Dual-Glo luciferase assay system obtained from Promega Corporation using Victor2 (PerkinElmer, Waltham, MA). Normalized firefly luciferase activity (firefly luciferase activity/Renilla luciferase activity) for each construct was compared with that of the pmirGLO vector without insert (MOCK), and normalized-fold induction was determined.
Statistics.
In this study, statistical analyses were performed using GraphPad Prism software. The significance of the analysis of microarrays was performed using the Kaplan-Meier method. Student’s t test was used for paired observations if data followed a normal distribution to compare DES-induced apoptosis in T cells and expression and quantification of Fas and FasL expression in thymocytes. Differential (upregulated or downregulated) expression of miRs was analyzed using a two-sample t test method. Multiple comparisons were made using the one-way analysis of variance test and Tukey-Kramer multiple comparisons tests. A P value of ≤0.05 was considered to be statistically significant.
Results
DES Induces Alterations in Thymic Cellularity in Mothers and Prenatal Fetuses.
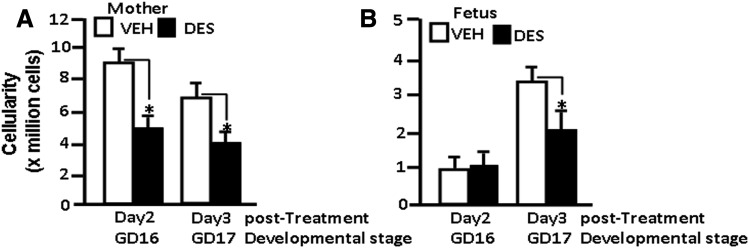
We have previously reported that prenatal exposure to DES induces thymic atrophy and apoptosis resulting from upregulation of Fas and Fas ligand expression (Brown et al., 2006b; Singh et al., 2012b). In this study, our goal was to investigate the role of microRNA in such immunologic changes induced by DES. To this end, we administered a single dose of DES (5 μg/kg) into pregnant C57BL/6 mice on GD 14 as described previously (Brown et al., 2006b; Singh et al., 2012b) and studied the effect on the thymocytes. On days 2 and 3 post-DES treatment (GD 16 and GD 17), the thymic cellularity of mothers and fetuses was determined. There was a significant decrease in the thymic cellularity of the mothers on both day 2 (GD 16) and day 3 (GD 17) post-DES exposure (Fig. 1A). In fetuses, however, no significant difference in thymic cellularity was observed on day 2 (GD 16), but a significant decrease in thymic cellularity of fetuses was observed on day 3 (GD 17) post-DES exposure (Fig. 1B). These data are consistent with previously published results from our laboratory that showed DES induces a decrease in thymic cellularity in both pregnant mothers and fetuses (Brown et al., 2006a,b; Camacho et al., 2004a,b). We also confirmed in these experiments that exposure to DES led to increased apoptosis in thymocytes from both mothers and fetuses and that DES-exposed cells had higher levels of Fas and FasL expression (data not shown), which is consistent with our earlier studies (Brown et al., 2006b; Singh et al. 2012b).
Fig. 1.
DES decreases thymic cellularity in both mothers and fetuses. DES (5 μg/kg of body weight) or VEH (control) was administered into pregnant mice (GD 14). The thymi from mothers and their fetuses were harvested on days 2 (GD 16) and 3 (GD 17) post-DES or post-VEH treatment to determine thymic cellularity. (A) DES-induced changes in thymic cellularity of mothers. (B) DES-induced changes in thymic cellularity of fetuses. The bars represent mean ± S.E.M. from groups of five mice (P > 0.05). The experiment was repeated thrice with consistent results.
Analysis of DES-Regulated miR Profile in Thymocytes from the Mother and Fetus.
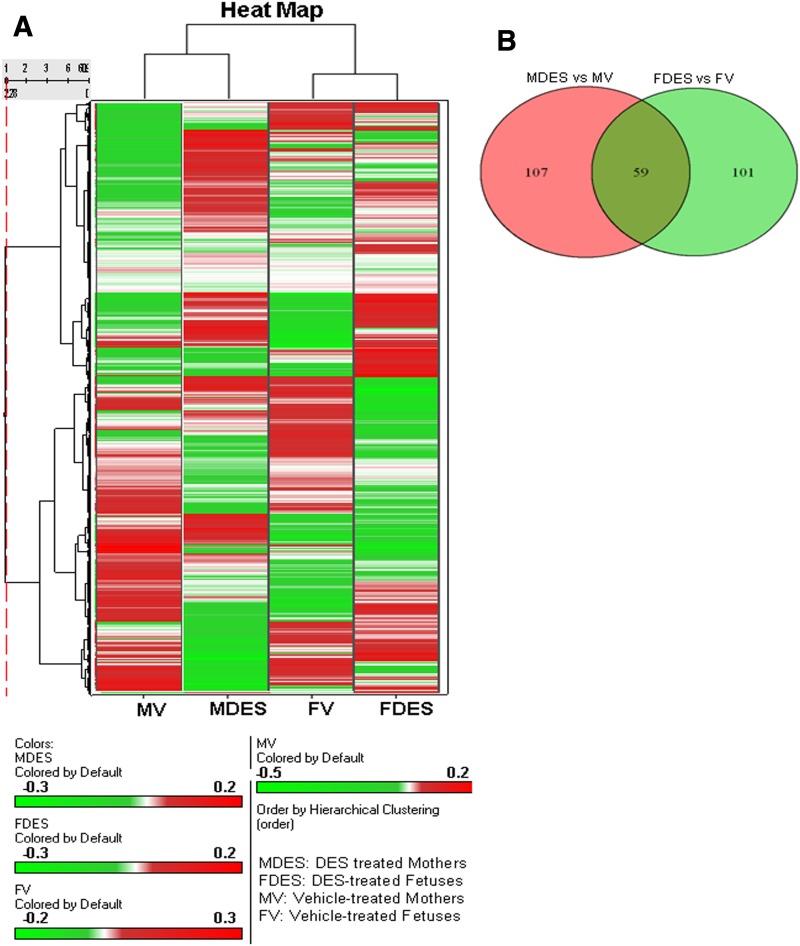
The data obtained from miR arrays of the thymocytes post-DES exposure were further analyzed. To this end, cluster analyses of 609 miRs (Fig. 2A) were performed using Ward’s method. Similarly, the measure of the miRs of the four groups [mother + VEH, mother + DES, fetus + VEH, and fetus + DES] was done using the half-square Euclidean distance method and ordering the function of miRs was done on the basis of input rank. As shown in Fig. 2A, visualization of the cluster analysis of miRs has been presented as a dendrogram and their expression pattern is reflected. Upon comparison of more than 1-fold dysregulated (upregulated or downregulated) miRs in DES-treated mothers versus VEH-treated mothers or DES-treated fetuses versus VEH-treated fetuses, there were 59 miRs that were common for both mothers and fetuses, whereas there were 107 miRs that were specific to mothers only and 101 miRs that were specific to fetuses only (Fig. 2B).
Fig. 2.
Heat map of miR expression profile in thymi of mothers and fetuses postexposure to DES. Total RNAs, including miRs from thymi of mothers and fetuses post-DES or post-VEH exposure were isolated. An miR array specific to the mouse was performed on an Affymetrix GeneChip miR platform. (A) Heat map depicting miR expression profile in thymi of mothers and prenatal fetuses exposed to DES or VEH (control). The expression pattern (green to red) represents the spectrum of downregulated to upregulated expression pattern of miRs. (B) Venn diagram showing relationship among various miRs differentially expressed in thymi of mothers treated with DES when compared with VEH and miRs differentially expressed in thymi of fetuses treated with DES when compared with VEH.
Differential Expression of miRs in Fetal Thymocytes.
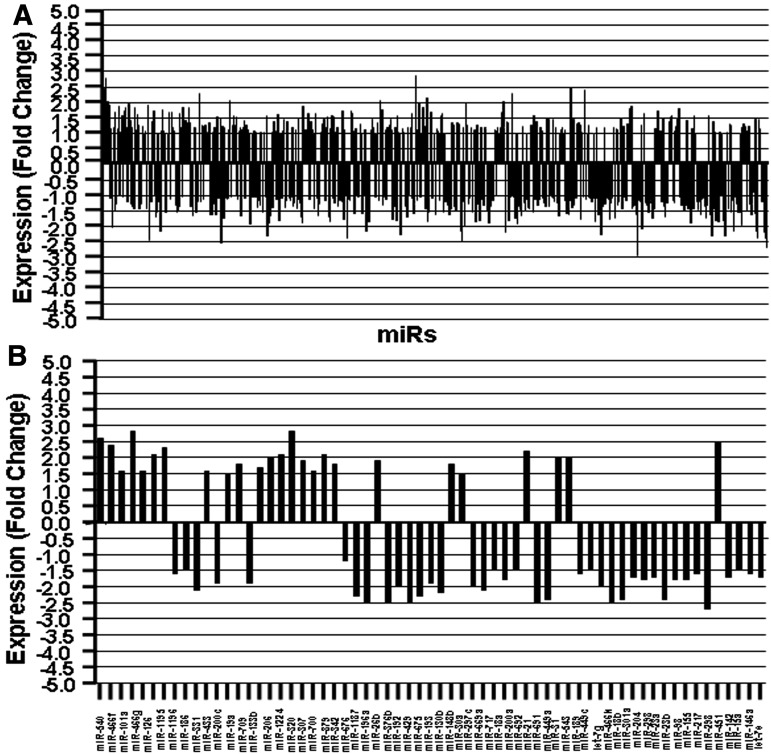
For all further studies, we focused on the miR profiles seen in fetal thymocytes to investigate if their expression correlated with thymic atrophy. To that end, we analyzed the differential (upregulated or downregulated) expression of miRs in fetal thymocytes using a two-sample t test method. The significance of the analysis of microarrays was performed using the Kaplan-Meier method. A P value of <0.01 in the t test was considered significant. As shown in Fig. 3, of the total 609 miRs screened, there were 63 miRs showing more than a 1.5-fold change (Fig. 3B), of which 34 miRs showed upregulated expression and 29 miRs showed downregulated expression in DES-exposed fetal thymocytes when compared with vehicle controls. Upon analysis of upregulated (34) miRs, 15 miRs showed expression from 1.5- to 1.9-fold, whereas 19 miRs showed 2-fold or more than 2-fold expression in DES-exposed thymocytes. Similarly, upon examination of downregulated (29) miRs following DES treatment, there are 19 miRs that showed expression from 1.5- to 1.9-fold and 10 miRs that showed expression of 2-fold or more than 2-fold (Supplemental Table 3). Change in the expression of miRs, 1.5-fold or higher, was considered positive, and thus, for all further analyses, we used miRs showing such changes.
Fig. 3.
Expression profile of miRs in thymi of fetuses postprenatal exposure to DES. Data obtained from miR arrays were analyzed for the expression of miRs. Dysregulation (fold change) of miR expression in DES-treated thymi was compared with miR expression in VEH-treated thymi. (A) depicts fold change expression profile of miRs post-DES exposure in comparison with VEH. (B) A significant number of miRs showed more than a 1.5-fold change (upregulated or downregulated) in their expression profile.
Validation of miR Expression by Real-Time PCR.
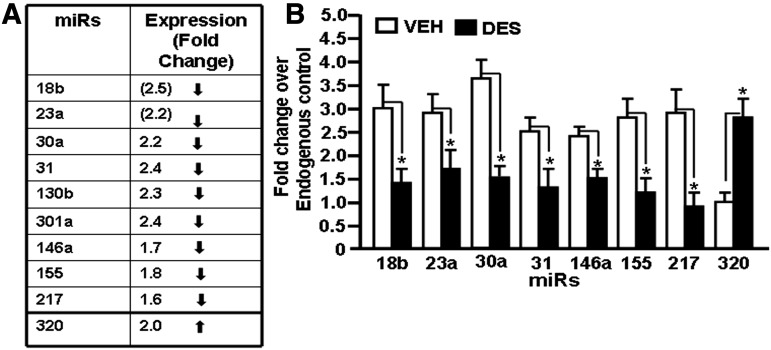
Based on the analysis of miR array data, we randomly chose eight miRs (seven downregulated miRs, miR-18b, miR-23a, miR-30a, miR-31, miR-146a, miR-155, and miR-217, and one upregulated miR, miR-320) to verify and validate their expression in fetal thymocytes post-DES or vehicle exposure (Fig. 4A). To this end, real-time PCR was performed on cDNAs converted from total RNAs, including miRs from thymocytes treated with DES or vehicle, as described in Materials and Methods. Data obtained from real-time PCR demonstrated downregulated expression of miR-18b, miR-23a, miR-30a, miR-31, miR-146a, miR-155, and miR-217 and upregulated expression of miR-320 in fetal thymocytes exposed to DES when compared with vehicle-treated thymocytes (Fig. 4B). Thus, the real-time PCR data validated the expression profile of selected miRs obtained from miR arrays.
Fig. 4.
Validation of expression profile of selected miRs in fetal thymi postprenatal exposure to DES. Based on the expression profile obtained from miR arrays, eight miRs (seven downregulated and one upregulated) were selected for validation. Real-time PCR using mouse-specific miR assays was performed. In (A), the miR expression profile from miR arrays is depicted. (B) Expression profile of selected miRs (miR-18b, miR-23a, miR-30a, miR-31, miR-146a, miR-155, miR-217, and miR-320) in fetal thymi post-DES exposure was determined using miR-specific primers and by performing real-time PCR. Data are depicted as mean ± S.E.M. of three independent experiments. The asterisk (*) in (B) indicates a statistically significant (P < 0.05) difference between the groups compared.
DES-Regulated miRs Play an Important Role in Various Disease Pathways.
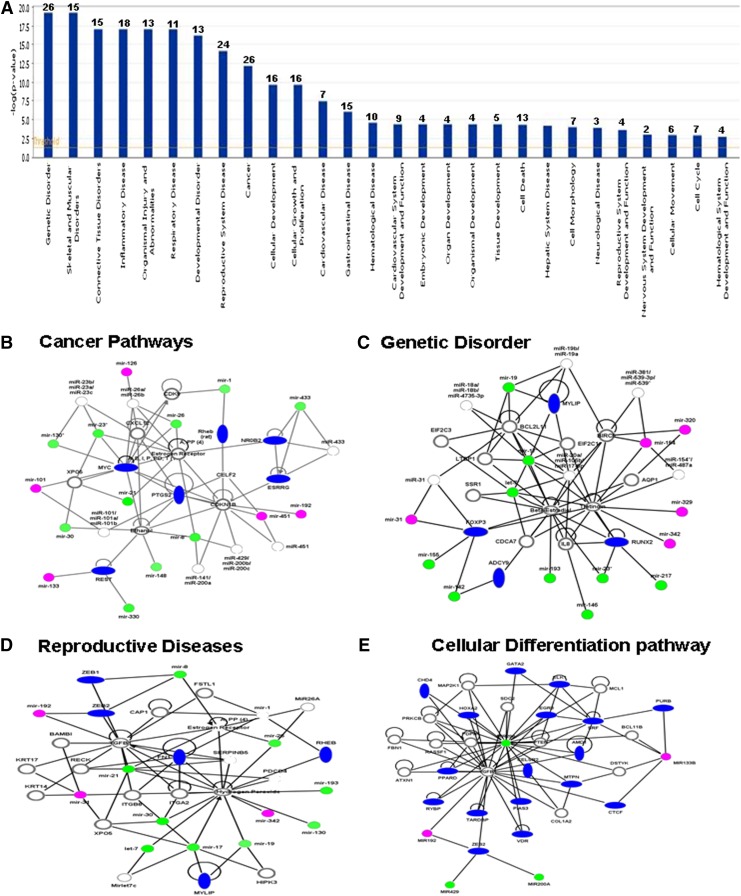
We next analyzed how DES-induced alterations in the expression of miRs in the fetus would affect various disease pathways. To this end, 63 miRs were analyzed, which showed a 1.5 or greater fold change, using IPA software and the database of the company (Ingenuity Inc.). Although, there were as many as 28 pathways that were found to be affected by the altered expression of miRs, there were at least 12 dominant pathways that were significantly affected (Fig. 5A). These included genetic disorder (26 miRs), skeletal and muscular disorder (15 miRs), connective tissue disorder (15 miRs), inflammatory diseases (18 miRs), organismal injury (13 miRs), respiratory diseases (11 miRs), developmental disorder (13 miRs), and cancer (26 miRs) (Fig. 5A). Similarly, as shown in Fig. 5A, there were several miRs involved in other pathways, including cellular development (16 miRS), cellular growth and proliferation pathways (16 miRs), cardiovascular diseases (seven miRs), gastrointestinal diseases (15 miRs), hematologic disease (10 miRs), endocrine development (four miRs), organ development (four miRs), organismal development (four miRs), tissue development (five miRs), cell death (13 miRs), and cell cycle (seven miRs). Upon further analysis of DES-regulated miRs (>1.5 fold upregulated or downregulated expression) using Ingenuity IPA software, the following pathways were found to be more significantly affected, including the cancer pathway (Fig. 5B), genetic disorder pathway (Fig. 5C), reproductive diseases pathway (Fig. 5D), and cellular differentiation pathways (Fig. 5E).
Fig. 5.
DES-regulated miRs and their association with functional networks and various pathways. DES-induced upregulated or downregulated (more than a 1.5-fold change) miRs were analyzed using IPA software and database of Ingenuity Systems (QIAGEN). The data presented in the graph demonstrate various pathways regulated by DES-induced miRs. The y-axis, –log(P value), represents the significance of function by random chance (IPA software, Ingenuity Systems, QIAGEN). The number over each bar represents the number of miRs involved in the pathways. DES-regulated miRs as described in (A) were further analyzed using IPA software and the database (Ingenuity Systems, QIAGEN). miRs involved in cancer pathway (B), genetic disorder pathway (C), reproductive pathway (D), and cellular differentiation pathway (E). In (B–E), thin line empty circles represents mature miRs with various functions, thick line empty circles represent various genes, magenta circles represent upregulated mature miRs, green circles represent downregulated mature miRs, and blue ovals represent various genes involved in the pathways.
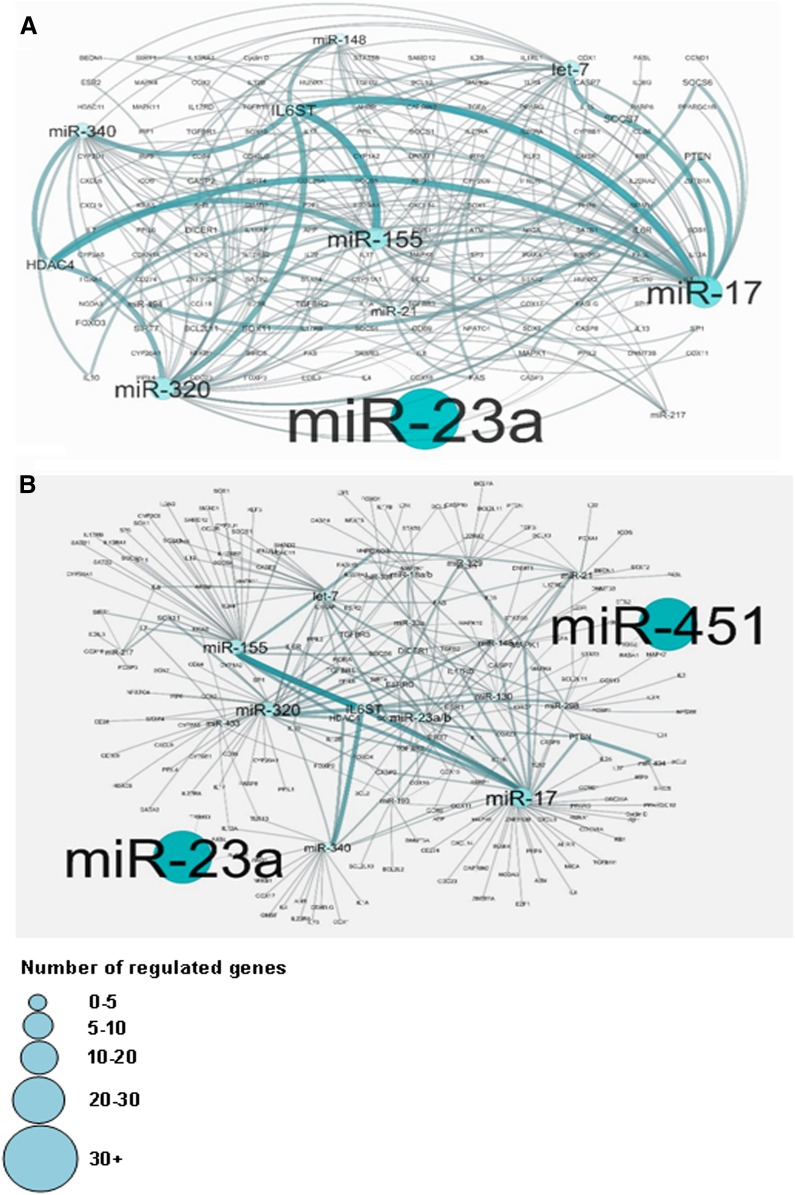
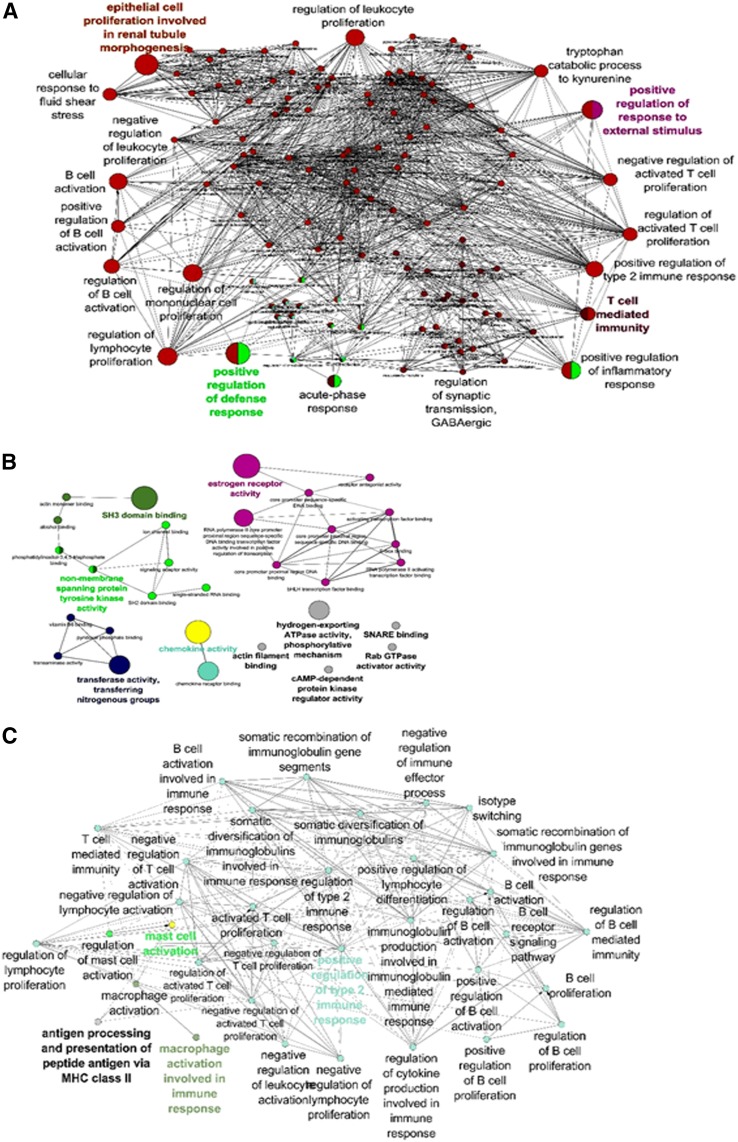
Upon further analysis of DES-regulated miRs and their relationship with various genes using the Cytoscape and Cluego software, there were large numbers of miRs that regulated various cytokine genes (Figs. 6 and 7) and genes involved in molecular pathways (Fig. 7B). These data together demonstrated that DES-induced alterations in the expression of miRs in the fetus may play a role in affecting various pathways and genes that are important in biologic, immunologic, and molecular functions of cells.
Fig. 6.
Potential gene targets of DES-regulated miRs. First, potential gene targets of DES-regulated miRs were analyzed by Ingenuity IPA software online (QIAGEN) and then the relationship between various DES-regulated miRs and genes were analyzed using Cytoscape version 3.0.1. (A and B) Data showing relationship between various DES-regulated miRs and genes post-Cytoscape 3.0.1 software analysis. The various sizes of miR boxes indicate the number of possible regulated genes by the particular miR.
Fig. 7.
Mapping of miRs and possible pathways. DES-regulated miRs and selected target genes were analyzed using the Cytoscape suite with ClueGo plugins. (A) demonstrates the biologic pathway, (B) demonstrates the molecular pathway, and (C) demonstrates the immunologic pathway regulated by miRs and associated genes.
Analysis of miRs Associated with Fas and FasL Expression.
Previous studies from our laboratory have reported that DES induces an increased expression of Fas and FasL in thymocytes, which plays a critical role in the induction of apoptosis (Brown et al., 2006a,b). In this context, we investigated potential miRs involved in the expression of Fas and FasL. We used TargetScanMouse 5.2, miRWalk, and microRNA.org software, and detected a significant binding affinity of miR-23a and miR-18b with the 3′UTR region of Fas and FasL genes, respectively (Supplemental Table 2).
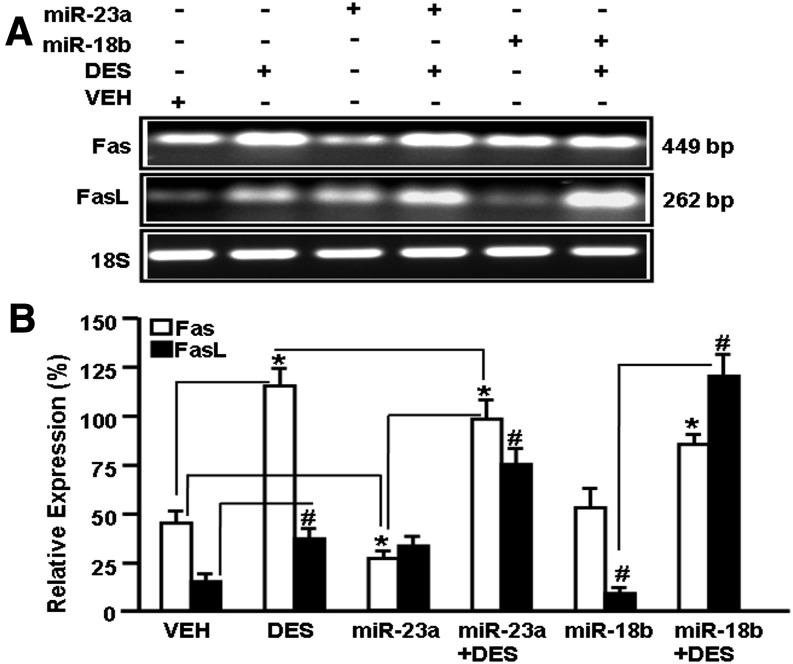
To confirm this analysis, we used EL4 T cells not transfected or transfected with mature miR-23a or miR-18b and cultured them in the absence or presence of DES for 24 hours. The expression of Fas and FasL was determined by performing RT-PCR. EL4 cells spontaneously expressed Fas and FasL (VEH treated), and treatment with DES further augmented their expression (Fig. 8). Interestingly, expression of Fas in EL4 cells transfected with miR-23a was decreased significantly when compared with VEH-treated EL4 cells (Fig. 8). Moreover, EL4 cells transfected with miR-23a and treated with DES also showed a decrease in Fas expression when compared with DES-treated cells (Fig. 8). Similarly, upregulated expression of FasL was observed in EL4 cells treated with DES when compared with VEH-treated cells (Fig. 8). Upon transfection of EL4 cells with miR-18b, there was significant downregulation of FasL expression, but upon DES treatment, downregulation of FasL expression was significantly reversed (Fig. 8). Overall, in these studies, we also noted that transfection of vehicle-treated EL4 cells with miR-23a decreased the expression of only Fas but not FasL, and similarly, transfection with miR-18b decreased the expression of FasL but not Fas. Also, DES reversed the miR transfection-induced inhibition of the expression of Fas and FasL. These data together suggested that DES-induced decrease in the expression of miR-23a and miR-18b may indeed be the mechanism through which Fas and FasL, respectively, get upregulated.
Fig. 8.
Expression of Fas and FasL in EL4 cells in the presence or absence of miR-23a or miR-18b post-DES treatment. Expression of Fas and FasL was determined by performing RT-PCR on cDNAs generated from total RNAs isolated from EL4 cells not transfected or transfected with mature miR-23a or mature miR-18b and treated with VEH or DES. 18S was used as an internal control. (A) RT-PCR data for Fas and FasL expression are presented. EL4 cells not transfected or transfected with mature miR-23a or mature miR-18b exposed to DES or VEH were analyzed for the expression of Fas and FasL by performing RT-PCR. (B) RT-PCR data are presented as the percentage of 18S expression, with the latter being considered as 100%. Data are depicted as mean ± S.E.M. of three independent experiments. The asterisk (*) in (B) indicates a statistically significant (P < 0.05) difference between the groups compared.
Association of DES-Induced Downregulation of miR-23a and miR-18b and Expression of Fas and FasL.
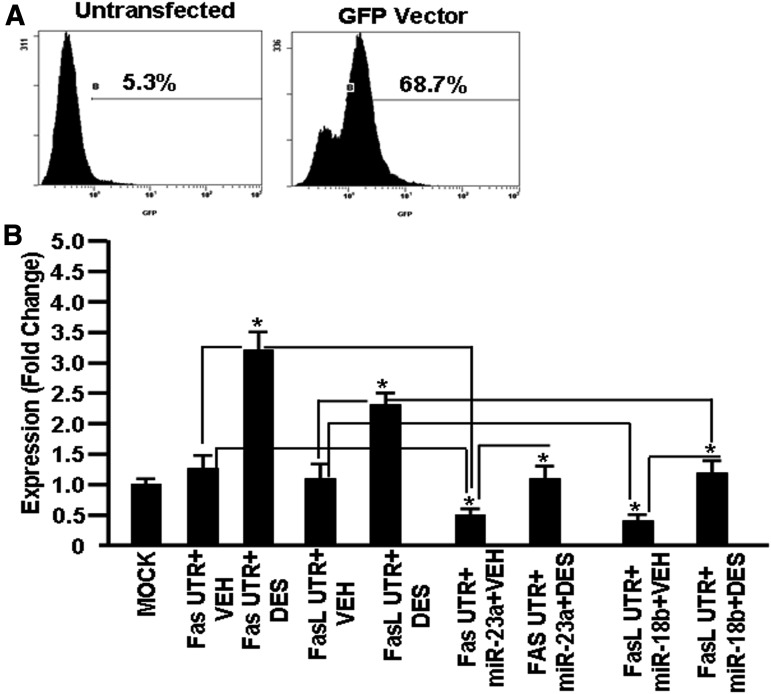
To further examine if DES mediated its effect through downregulation of miR-23a and miR-18b, Fas UTR containing the miR-23a binding region (Fig. 9A) or FasL UTR region containing the miR-18b binding region (Fig. 9B) was cloned into the pmiRGLO luciferase expression vector and the clones were designated as pmirGLO–Fas UTR and pmirGLO–FasL UTR. EL4 cells transfected with pmiRGLO without insert (MOCK), pmirGLO–Fas UTR, pmirGLO–Fas UTR + mature miR-23a, pmirGLO–FasL UTR, or pmirGLO–FasL UTR + mature miR-18b were next treated with VEH or DES for 24 hours. EL4 cells were also transfected with a vector containing green fluorescent protein as a positive control for transfection. There was more than 68% transfection of EL4 cells (Fig. 10A). Upon analysis of luciferase expression in the presence of the Fas or FasL UTR region, there was a significantly upregulated expression of luciferase (3–3.5 fold) in EL4 cells transfected with pmirGLO–Fas UTR in the presence of DES when compared with EL4 cells transfected with pmirGLO–Fas UTR but treated with VEH (Fig. 10B). Similarly, luciferase expression was upregulated (2–2.5 fold) in EL4 cells transfected with pmirGLO–FasL UTR and treated with DES when compared with similar cells treated with VEH (Fig. 10B). Next, we examined luciferase expression in EL4 cells that were transfected with either pmirGLO–Fas UTR + mature miR-23a or pmirGLO–FasL UTR + mature miR18b in the absence or presence of DES. The expression of luciferase was decreased in EL4 cells with pmirGLO–Fas UTR + mature miR-23a + DES when compared with cells with pmirGLO–Fas UTR + DES (Fig. 10B). Similar results were seen in the luciferase expression in EL4 cells transfected with pmirGLO–FaL UTR + mature miR-18b + DES when compared with cells with pmirGLO–FasL UTR + DES (Fig. 10B). These data demonstrated that DES regulates the expression of Fas and FasL, at least in part through downregulation of miR-23a and miR-18b expression, respectively.
Fig. 9.
Physical map of Fas and FasL UTR showing miR-23a and miR-18b binding sites. (A) Physical map of Fas UTR region demonstrating the binding site of miR-23a and the position of the primer pairs in Fas UTR. (B) Physical map of FasL UTR region demonstrating the binding site of the miR-18b region and position of the primer pairs in FasL UTR.
Fig. 10.
Expression of luciferase in EL4 cells in the presence or absence of Fas UTR containing the miR-23a binding site or FasL UTR containing the miR-18b binding site post-VEH or post-DES treatment. EL4 cells were transfected with pmiRGLO without an insert (MOCK), pmiRGLO–Fas UTR, pmiRGLO–Fas UTR + mature miR-23a, pmiRGLO–FasL UTR, or pmiRGLO–FasL UTR + mature miR-18b. EL4 cells were also transfected with a vector containing green fluorescent protein (GFP). Forty-eight hours post-transfection, cells were examined for GFP expression by flow cytometry for transfection efficiency. (A) Luciferase assays were performed to determine luciferase expression in EL4 cells post-transfection. (B) Luciferase data are presented as a fold change in expression. Data are depicted as mean ± S.E.M. of three independent experiments. The asterisk (*) in (B) indicates a statistically significant (P < 0.05) difference between the groups compared.
Discussion
Exposure to DES during pregnancy in humans has been shown to trigger a number of clinical disorders both in the mother as well as the developing fetus well into adulthood (Herbst, 1981; Palmer et al., 2005; Goodman et al., 2011; Hoover et al., 2011). Prenatal exposure to DES has also been shown to cause significant alterations in the immune response, including altered susceptibility to autoimmune diseases, cancer, and infections (Maier et al., 1985; Marselos and Tomatis, 1992a,b; Holladay et al., 1993; Giusti et al., 1995; Laitman, 2002; Besteman et al., 2005; Brown et al., 2006a; Singh et al., 2012b; Hilakivi-Clarke et al., 2013). Although in recent years, miRs have been the focus of several studies due to their direct role in gene expression, there is not much information in the literature regarding DES-mediated effects on gene expression and function regulated by miRs. Therefore, in the present study, we investigated whether exposure to DES during pregnancy alters the miR profile in the pregnant mother as well as the fetus. We noted that while DES caused similar alterations in the mother and fetus as 59 miRs showing similar changes, there were significant numbers of miRs that were uniquely altered in these two groups. The differences in miR expression between the mother and fetus may result from the differential amounts of DES exposure between the mother and fetus. This may be because of direct exposure of the mother’s thymocytes to DES, whereas the fetal thymocytes may be exposed to the amounts that cross the placenta to reach the fetus. In addition, while the mother has a mature thymus, the fetus, on the other hand, has an immature thymus that may be more susceptible to DES toxicity.
Cluster analysis of the miR expression profile showed clustering of a large number of miRs that were differentially expressed in fetal thymi upon exposure to DES. We identified several upregulated or downregulated miRs in the presence of DES. We validated the expression profile of some of the miRs (miR-18b, miR-23a, miR-30a, miR-31, miR-130b, miR-146a, miR-155, miR-217, miR-301a, and miR-320) by performing real-time PCR, and the data confirmed and corroborated with the miR expression profile data obtained from an miR arrays analysis. Furthermore, we also verified the relationship of selected miRs (miR-23a and miR-18b) and their target gene expression. We selected miR-23a and miR-18b because miR-23a was found to have a binding affinity with mouse Fas UTR, whereas miR-18b had a binding affinity with mouse FasL UTR, and both miRs were downregulated in fetal thymi post-DES exposure (Fig. 4B). Moreover, previous studies from our laboratory showed that DES caused an increased expression of Fas and FasL expression in thymocytes of pregnant mothers as well as the fetus (Brown et al., 2006a,b). Examination of Fas and FasL expression by RT-PCR, on the other hand, showed an increased expression in fetal thymi upon exposure to DES when compared with VEH (Fig. 8).
As negative regulators of gene expression, the expression profile of miRs in the thymus is very important. In this study, we considered DES-regulated miR expression to be significant when it was altered by more than 1.5-fold, as was also reported in other studies (Moffat et al., 2007). The downregulated expression of several miRs (miR-18a, miR-18b, miR-23a, miR-23b, miR-26b, miR-30a, miR-31, miR-98, miR-130b, miR-146a, miR-155, miR-204, miR-217, miR-298, miR-301a, and miR-320) in fetal thymi post-DES exposure indicated that the expression of respective genes that they regulate may be increased. Upon closer analysis of the miR-gene relationship, we observed that miR-18a and miR-18b possessed a binding affinity with FasL 3′UTR, whereas miR-23a and miR-23b possessed a binding affinity with Fas 3′UTR. Similarly, miR-146a possessed a binding affinity with BRCA1 3′UTR, miR-155 possessed a binding affinity with SHIP1 3′UTR, miR-217 possessed a binding affinity with Foxp3 3′UTR, and miR-298 possessed a binding affinity with BACE1 3′UTR. Thus, downregulated expression of these miRs post-DES exposure suggests that they may be involved in the regulation of many genes, including apoptosis in fetal thymi.
In the current study, we also noted downregulated expression of miR-31, miR-34b, and miR-181a in fetal thymi post-DES exposure. Upon analysis, miR-31 showed a binding affinity with the 3′UTR region of the Foxp3 and CYP1A1 genes. Foxp3 expression is known to be expressed predominantly in regulatory T cells (Biller et al., 2007; Ruan et al., 2009). The computational analysis of miR-34b and miR-181a showed a binding affinity with 3′UTR of the Notch ligand JAG-1 and a large number of zinc finger genes, respectively. Expression of miR-34b has been shown to be regulated by p53, which mediates cell cycle arrest and promotes apoptosis (Hermeking, 2007, 2010). Also, overexpression of miR-34b has been shown to decrease the expression of a number of cell cycle regulatory proteins, including cyclin D1, c-MET, and CDK4 (Hermeking, 2007, 2010), and thus hampers cell cycle progression (Hermeking, 2007, 2010). miR-34b has been reported to act as a tumor suppressor in colorectal cancer (Wu et al., 2014), prostate cancer (Geier et al., 2010), and gastric cancer (Wang et al., 2014; Yang et al., 2014). In another recent study, Lee et al. have shown the role of miR-34b in the expression of estrogen receptor and estrogen receptor–mediated growth of breast cancer cells in vitro (Lee et al., 2011). These data are consistent with our previous findings that DES alters the T cell selection process in the thymus (Brown et al., 2006a). It has also been demonstrated that miR-181a modulates the expression of zinc finger family member genes by directly targeting their coding regions (Huang et al., 2010). Together, these data demonstrated that the miRs that are downregulated in fetal thymi by DES may control the expression of genes involved in various mechanisms and functions like T cell selection in the thymus, thymic atrophy, immunosuppression, and toxicity. This may explain how DES may alter the positive and negative selection of T cells in the thymus, as reported by us previously (Brown et al., 2006a).
We also noted DES induced upregulation of several miRs (miR-21, miR-101a, miR-126, miR-133b, miR-141, miR-200a, miR-200b, miR-320, miR-429, miR-451, miR-466f, and miR-466g). These miRs regulate the expression of various genes that control different physiologic and biologic mechanisms and functions. For example, miR-21, which showed increased expression (>2.3-fold) in fetal thymi post-DES exposure, plays an important role in cancer development (Jazbutyte and Thum, 2010). miR-21 expression has been shown to be activated in multiple types of cancers, such as breast, liver, brain, prostate, myometrial cancers (Jazbutyte and Thum 2010). Thus, miR-21 regulates a large number of target proteins that may be involved in cellular survival, apoptosis, and cell invasiveness (Jazbutyte and Thum 2010). Tanaka et al. (2009) reported that miR-101a controls mammary gland development in mice through alterations in the expression of Cox-2. Several miRs, such as miR-141, miR-200a, and miR-200b, were upregulated in fetal thymi post-DES exposure. These miRs play a significant role in ovarian tumorigenesis and cancer development. In a recent study, it was shown that miR-141 and miR-200a affect ovarian tumorigenesis by controlling the oxidative stress response (Mateescu et al., 2011). Furthermore, there were several miRs that were downregulated (varied from 1.5- to 2.5-fold) post-DES exposure. These miRs were miR-23a, miR-23b, miR-18b, miR-26b, miR-98, miR-148b, and miR-186 and are expressed in various tissues, such as breast, cartilage, endothelial cells, and embryonic tissues. These downregulated miRs have been shown to control genes that are involved in various physiologic functions in these tissues (Baltimore et al., 2008). Thus, overall, our miR data were consistent with DES-induced toxicity in reproductive organs and the ability to act as a carcinogen (Walker et al., 2013).
The current study suggests that prenatal exposure to environmental stressors can have a significant impact on the miR profile. For example, prenatal exposure to arsenic was shown to alter miR expression associated with innate and adaptive immune responses (Rager et al., 2014). Additionally, we have also shown that 2,3,7,8-tetrachlorodibenzo-p-dioxin altered the expression of miRs in thymuses when pregnant mothers were exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (Singh et al., 2012a). In summary, we demonstrate for the first time that prenatal exposure to DES can cause a significant effect on the miR profile in the thymuses of both the mother and fetus. These upregulated or downregulated miRs may influence the regulation of genes that affect the development of the immune cells and other organ systems. Identification of miRs as targets for DES-induced modulation of gene expression offers novel mechanisms to understand DES-regulated molecular mechanisms and its long-term effects.
Supplementary Material
Acknowledgments
The authors thank Drasti Patel for technical help.
Abbreviations
- DES
diethylstilbestrol
- FasL
Fas ligand
- GD
gestation day
- miR
microRNA
- RT-PCR
reverse-transcription polymerase chain reaction
- UTR
untranslated region
- VEH
vehicle
Authorship Contributions
Participated in research design: N. P. Singh, P. Nagarkatti, M. Nagarkatti.
Conducted experiments: N. P. Singh, Abbas, Menard, U. P. Singh.
Contributed new reagents or analytic tools: N. P. Singh, P. Nagarkatti, M. Nagarkatti.
Performed data analysis: N. P. Singh, Abbas, U. P. Singh, Zhang, P. Nagarkatti, M. Nagarkatti.
Wrote or contributed to the writing of the manuscript: N. P. Singh, P. Nagarkatti, M. Nagarkatti.
Footnotes
This work was supported in part by National Institutes of Health National Center for Complementary and Alternative Medicine [P01-AT003961 and R01-AT006888], National Institute of Environment and Health Sciences [R01-ES019313], National Institute of Mental Health [R01-MH094755], and National Institute of General Medical Sciences [P20-GM103641]; Veterans Affairs Merit Award [BX001357]; and University of South Carolina ASPIRE 1 Grant [A011].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. (2008) MicroRNAs: new regulators of immune cell development and function. Nat Immunol 9:839–845. [DOI] [PubMed] [Google Scholar]
- Bartel DP. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. [DOI] [PubMed] [Google Scholar]
- Besteman EG, Zimmerman KL, Holladay SD. (2005) Diethylstilbestrol (DES)-induced fetal thymic atrophy in C57BL/6 mice: inhibited thymocyte differentiation and increased apoptotic cell death. Int J Toxicol 24:231–239. [DOI] [PubMed] [Google Scholar]
- Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW. (2007) Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol 116:69–78. [DOI] [PubMed] [Google Scholar]
- Brown N, Nagarkatti M, Nagarkatti PS. (2006a) Diethylstilbestrol alters positive and negative selection of T cells in the thymus and modulates T-cell repertoire in the periphery. Toxicol Appl Pharmacol 212:119–126. [DOI] [PubMed] [Google Scholar]
- Brown N, Nagarkatti M, Nagarkatti PS. (2006b) Induction of apoptosis in murine fetal thymocytes following perinatal exposure to diethylstilbestrol. Int J Toxicol 25:9–15. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS. (2004a) Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on maternal immune response during pregnancy. Arch Toxicol 78:290–300. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS. (2004b) Evidence for induction of apoptosis in T cells from murine fetal thymus following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci 78:96–106. [DOI] [PubMed] [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. (2010) In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer 1:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MT, Nagarkatti M, Nagarkatti PS. (2004) Combined screening of thymocytes using apoptosis-specific cDNA array and promoter analysis yields novel gene targets mediating TCDD-induced toxicity. Toxicol Sci 78:116–124. [DOI] [PubMed] [Google Scholar]
- Ford CD, Johnson GH, Smith WG. (1983) Natural killer cells in in utero diethylstilbesterol-exposed patients. Gynecol Oncol 16:400–404. [DOI] [PubMed] [Google Scholar]
- Frawley R, White K, Jr, Brown R, Musgrove D, Walker N, Germolec D. (2011) Gene expression alterations in immune system pathways in the thymus after exposure to immunosuppressive chemicals. Environ Health Perspect 119:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier R, Adler S, Rashid G, Klein A. (2010) The synthetic estrogen diethylstilbestrol (DES) inhibits the telomerase activity and gene expression of prostate cancer cells. Prostate 70:1307–1312. [DOI] [PubMed] [Google Scholar]
- Giusti RM, Iwamoto K, Hatch EE. (1995) Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med 122:778–788. [DOI] [PubMed] [Google Scholar]
- Goodman A, Schorge J, Greene MF. (2011) The long-term effects of in utero exposures—the DES story. N Engl J Med 364:2083–2084. [DOI] [PubMed] [Google Scholar]
- Hegde VL, Tomar S, Jackson A, Rao R, Yang X, Singh UP, Singh NP, Nagarkatti PS, Nagarkatti M. (2013) Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Δ9-tetrahydrocannabinol in vivo: regulation of CCAAT/enhancer-binding protein α by microRNA-690. J Biol Chem 288:36810–36826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AL. (1981) The current status of the DES-exposed population. Obstet Gynecol Annu 10:267–278. [PubMed] [Google Scholar]
- Hermeking H. (2010) The miR-34 family in cancer and apoptosis. Cell Death Differ 17:193–199. [DOI] [PubMed] [Google Scholar]
- Hermeking H. (2007) p53 enters the microRNA world. Cancer Cell 12:414–418. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, de Assis S, Warri A. (2013) Exposures to synthetic estrogens at different times during the life, and their effect on breast cancer risk. J Mammary Gland Biol Neoplasia 18:25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay SD, Blaylock BL, Comment CE, Heindel JJ, Fox WM, Korach KS, Luster MI. (1993) Selective prothymocyte targeting by prenatal diethylstilbesterol exposure. Cell Immunol 152:131–142. [DOI] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, et al. (2011) Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 365:1304–1314. [DOI] [PubMed] [Google Scholar]
- Huang S, Wu S, Ding J, Lin J, Wei L, Gu J, He X. (2010) MicroRNA-181a modulates gene expression of zinc finger family members by directly targeting their coding regions. Nucleic Acids Res 38:7211–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Croce CM. (2009) MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 27:5848–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbutyte V, Thum T. (2010) MicroRNA-21: from cancer to cardiovascular disease. Curr Drug Targets 11:926–935. [DOI] [PubMed] [Google Scholar]
- Laitman CJ. (2002) DES exposure and the aging woman: mothers and daughters. Curr Womens Health Rep 2:390–393. [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854. [DOI] [PubMed] [Google Scholar]
- Lee YM, Lee JY, Ho CC, Hong QS, Yu SL, Tzeng CR, Yang PC, Chen HW. (2011) miRNA-34b as a tumor suppressor in estrogen-dependent growth of breast cancer cells. Breast Cancer Res 13:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier DB, Newbold RR, McLachlan JA. (1985) Prenatal diethylstilbestrol exposure alters murine uterine responses to prepubertal estrogen stimulation. Endocrinology 116:1878–1886. [DOI] [PubMed] [Google Scholar]
- Marselos M, Tomatis L. (1992a) Diethylstilboestrol: I, pharmacology, toxicology and carcinogenicity in humans. Eur J Cancer 28A:1182–1189. [DOI] [PubMed] [Google Scholar]
- Marselos M, Tomatis L. (1992b) Diethylstilboestrol: II, pharmacology, toxicology and carcinogenicity in experimental animals. Eur J Cancer 29A:149–155. [DOI] [PubMed] [Google Scholar]
- Mateescu B, Batista L, Cardon M, Gruosso T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P, Sastre-Garau X, et al. (2011) miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med 17:1627–1635. [DOI] [PubMed] [Google Scholar]
- Moffat ID, Boutros PC, Celius T, Lindén J, Pohjanvirta R, Okey AB. (2007) microRNAs in adult rodent liver are refractory to dioxin treatment. Toxicol Sci 99:470–487. [DOI] [PubMed] [Google Scholar]
- Noller KL, Blair PB, O’Brien PC, Melton LJ, 3rd, Offord JR, Kaufman RH, Colton T. (1988) Increased occurrence of autoimmune disease among women exposed in utero to diethylstilbestrol. Fertil Steril 49:1080–1082. [DOI] [PubMed] [Google Scholar]
- Ohta R, Ohmukai H, Toyoizumi T, Shindo T, Marumo H, Ono H. (2014) Ovarian dysfunction, obesity and pituitary tumors in female mice following neonatal exposure to low-dose diethylstilbestrol. Reprod Toxicol 50:145–151. [DOI] [PubMed] [Google Scholar]
- Okada A, Sato T, Ohta Y, Buchanan DL, Iguchi T. (2001) Effect of diethylstilbestrol on cell proliferation and expression of epidermal growth factor in the developing female rat reproductive tract. J Endocrinol 170:539–554. [DOI] [PubMed] [Google Scholar]
- Palmer JR, Wise LA, Robboy SJ, Titus-Ernstoff L, Noller KL, Herbst AL, Troisi R, Hoover RN. (2005) Hypospadias in sons of women exposed to diethylstilbestrol in utero. Epidemiology 16:583–586. [DOI] [PubMed] [Google Scholar]
- Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, Drobná Z, Currier J, Douillet C, Olshan AF, et al. (2014) Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen 55:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. (2009) Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity 31:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Nagarkatti M, Nagarkatti P. (2008) Primary peripheral T cells become susceptible to 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated apoptosis in vitro upon activation and in the presence of dendritic cells. Mol Pharmacol 73:1722–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Guan H, Nagarkatti P, Nagarkatti M. (2012a) Prenatal exposure to TCDD triggers significant modulation of microRNA expression profile in the thymus that affects consequent gene expression. PLoS ONE 7:e45054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M. (2012b) Prenatal exposure of mice to diethylstilbestrol disrupts T-cell differentiation by regulating Fas/Fas ligand expression through estrogen receptor element and nuclear factor-κB motifs. J Pharmacol Exp Ther 343:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N. (1963) Vaginal cornification in persistent-estrous mice. Endocrinology 72:607–619. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Haneda S, Imakawa K, Sakai S, Nagaoka K. (2009) A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation 77:181–187. [DOI] [PubMed] [Google Scholar]
- Walker LM, Tran S, Robinson JW. (2013) Luteinizing hormone—releasing hormone agonists: a quick reference for prevalence rates of potential adverse effects. Clin Genitourin Cancer 11:375–384. [DOI] [PubMed] [Google Scholar]
- Wang AM, Huang TT, Hsu KW, Huang KH, Fang WL, Yang MH, Lo SS, Chi CW, Lin JJ, Yeh TS. (2014) Yin Yang 1 is a target of microRNA-34 family and contributes to gastric carcinogenesis. Oncotarget 5:5002–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways SC, Mortola JF, Zvaifler NJ, Weiss RJ, Yen SS. (1987) Alterations in immune responsiveness in women exposed to diethylstilbestrol in utero. Fertil Steril 48:193–197. [DOI] [PubMed] [Google Scholar]
- Wu XD, Song YC, Cao PL, Zhang H, Guo Q, Yan R, Diao DM, Cheng Y, Dang CX. (2014) Detection of miR-34a and miR-34b/c in stool sample as potential screening biomarkers for noninvasive diagnosis of colorectal cancer. Med Oncol 31:894. [DOI] [PubMed] [Google Scholar]
- Yang C, Ma X, Liu D, Wang Y, Tang R, Zhu Y, Xu Z, Yang L. (2014) Promoter polymorphisms of miR-34b/c are associated with risk of gastric cancer in a Chinese population. Tumour Biol 35:12545–12554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.