Abstract
Management of psoriasis is a challenge to the treating physician. The chronic inflammatory state of psoriasis with exacerbations and remissions necessitate “on-and-off” treatment schedules. The safety profiles of drugs and tolerability issues for patients are important factors to be considered during treatment. Various biological agents targeting T-cells and the inflammatory cytokines are available for systemic treatment of psoriasis. However, major causes of concern while using these drugs are risk of susceptibility to infection and development of anti-drug antibodies, which will affect the pharmacokinetic properties, efficacy, and safety profile of the drug. Itolizumab, a humanized anti-CD6 monoclonal antibody, is a new molecule that acts by immunomodulating the CD6 molecule. CD6 is a co-stimulatory molecule required for optimal T-cell stimulation by the antigen-presenting cells. This step is crucial in T-cell proliferation to form Th1 and Th17 cells, which play a major role in the pathogenesis of psoriasis. This article deals with the properties of Itolizumab and its role in the treatment of psoriasis. Based on the available published data, Itolizumab seems to have a better adverse effects profile and at the same time comparatively less efficacy when compared to other biological agents available for treating psoriasis. Larger studies with longer duration are required to clearly depict the long-term side effects profile.
Keywords: Itolizumab, CD6, psoriasis, monoclonal antibody, biological
Introduction
Psoriasis is a chronic inflammatory disease of the skin characterized by exacerbations and remissions affecting 1%–3% of the world’s population, and approximately 20% of patients have moderate to severe disease.1,2 Psoriasis reduces the quality of life and brings about many psychological and psychosocial problems in the affected individual. Approximately 20% of patients are affected with moderate to severe type of psoriasis necessitating systemic therapy.2 The exact etiology remains unclear. However, it has been established that genetic predisposition and environmental stimuli synchronously contribute toward immunological, biochemical, and vascular abnormalities, leading to abnormal epidermal proliferation and differentiation. Recent studies show that, in addition to T lymphocytes, keratinocytes also play a major role in the cutaneous immune response of psoriasis.1,3
Various systemic drugs, including biological agents, are available for generalized, pustular, erythrodermic psoriasis and psoriatic arthritis. All of them have their own merits and demerits as far as efficacy and safety are concerned. Some of them cause T-cell depletion, which leads to increased susceptibility to infections4,5 and the risk of development of lymphomas6 later in life. This article deals with a new humanized monoclonal anti-CD6 antibody, Itolizumab, which has been found to be useful in the treatment of psoriasis because it has a better side effects profile.
Role of immune cells and co-stimulatory signals in pathogenesis of psoriasis
Pathogenesis of psoriasis is complex and involves genetic, environmental, and immunological factors. Consequent to extensive ongoing research in this field, new factors are constantly being added to the cascade of events that lead to the formation of psoriatic plaques.1–3 A brief note on the role of immune cells in the pathogenesis of psoriasis is mentioned in this section, in the paragraph below.
In a person who is genetically predisposed to develop psoriasis, the cells of the innate immune system (dendritic cells, histiocytes, keratinocytes, neutrophils, and monocytes) get activated and release cytokines such as TNF-α (tumor necrosis factor-alpha) and IL-1 (interleukin-1) when subjected to specific environmental stimuli.1,7 These cytokines activate the APCs (antigen-presenting cells). Activated APCs, along with the processed antigens, migrate to the regional lymph nodes where they activate the naïve T-cells. This will result in differentiation of naïve T-cells to T helper cells such as Th1, Th2, Th17, and T regulatory cells (Figure 1).1,3,9,10 The decreased activity of regulatory T-cells results in increased epidermal infiltration by CD4+ and CD8+ cells, leading to epidermal hyper-proliferation.2 The Th1 and Th17 cells produce inflammatory cytokines such as interferon gamma (IFN-γ), TNF-α, IL-6, IL-17, IL-22, etc. These cytokines bring about vasodilatation and expression of adhesion molecules on endothelial cells. This also leads to recruitment of inflammatory cells, leading to keratinocyte proliferation.11 Optimal T-cell stimulation by APCs requires at least two signals. The first signal is antigen-specific and involves interaction between TCR (T-cell receptor) and MHC (major histocompatibility complex) molecules on the APC membrane. The second signal is antigen non-specific and is provided by co-stimulatory signals. This involves interaction between receptors and ligands on APCs and T-cells.1,3 Activation of T-cells without co-stimulation leads to T-cell apoptosis or anergy, T-cell deletion, and to the development of immune tolerance.12 Anergic T-cells cannot proliferate upon re-stimulation.
Figure 1.
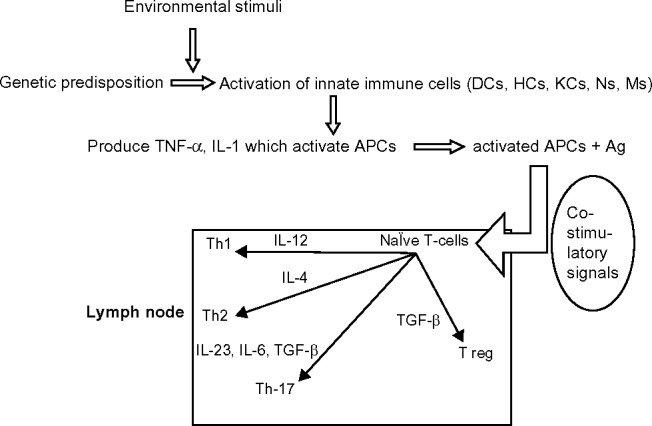
The activation of naïve T-cells to different types of T-cells by APCs in the lymph node. Successful T-cell activation occurs in the presence of co-stimulatory signals.
Abbreviations: Ag, antigen; DCs, dendritic cells; HCs, histiocytes; KCs, keratinocytes; Ns, neutrophils; Ms, monocytes; APCs, antigen-presenting cells; TNF, tumor necrosis factor; IL, interleukin; Th, helper T-cell; TGF, transforming growth factor; T reg, T regulatory cells.
The key pathogenic events in psoriasis include hyper-proliferation and differentiation of keratinocytes, activation of innate keratinocyte defense response, activation of transcription factors leading to increased levels of proinflammatory cytokines, dermal vascular proliferation, neutrophil chemotaxis, and finally sustainment of inflammation.1,3,7–9,13 The effect of various T-cells and pro-inflammatory cytokines in psoriasis has been summarized in Figure 2.
Figure 2.
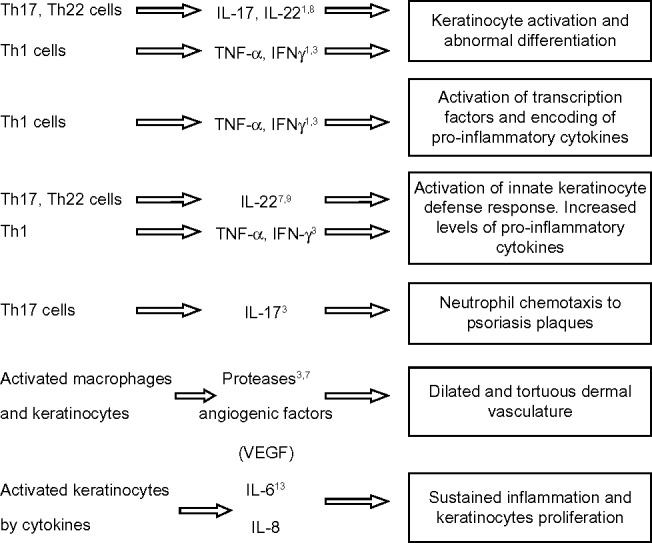
Types of T helper (Th) cells and pro-inflammatory cytokines responsible for major pathogenic events in psoriasis.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; VEGF, vascular endothelial growth factor; Th, helper T-cell.
The CD6 molecule and its role in the pathogenesis of psoriasis
CD6 is a 105–130 kDa surface glycoprotein9 found on mature T-cells, immature B-cells, the B1a subset of B-lymphocytes, and certain regions of the brain.10,14 It is thought to play a role in cell proliferation, adhesion, differentiation, and survival.15 It has three scavenger receptor cysteine-rich (SRCR) domains in its extracellular region.10,16 Studies have identified the presence of CD6 ligand, known as CD166 or activated leukocyte-cell adhesion molecule (ALCAM), expressed on T- and B-lymphocytes, APCs, thymocytes of the thymus, spleen, lymph nodes, and skin and neuronal cells.2,17,18 ALCAM binding is through the extracellular SRCR domain (D) 3 of CD6 and has an important role in T-cell and APC interactions.10,19 The CD6–ALCAM interaction contributes to the formation of immunological synapses. It facilitates stable adhesion between T-cells and APCs and has a role in T-cell differentiation, proliferation, and maturation.20 Apart from this, the CD6 co-stimulatory pathways enhance CD3 proliferation and increase in number of CD25 molecules. It also activates the release of TNF-α, IFN-γ, and IL-6. All these events result in tissue inflammation.20 The modulation of CD6-mediated activation of T-cells may also be related to reduction of intracellular phosphoproteins, which are involved in the intracellular signaling triggered by CD6.2
Itolizumab
Itolizumab is a humanized recombinant immunoglobulin G1 (IgG1) monoclonal antibody, which is a selective T-cell co-stimulation modulator, targeting the SRCR–D 1 of CD6.16,18,19,21 It has two heavy chains and two light chains with a disulfide bond. The molecular weight is 148 kDa and has 449 amino acids in the heavy chain and 214 amino acids in the light chain.2,16,18 Itolizumab is commercially available as preservative-free, single-use 25 mg/5 mL vials for intravenous (IV) injection (brand name Alzumab™; Biocon Ltd, Bangalore, India). It is a clear, colorless buffer solution with a pH of 7.0±0.5.18
The parent antibody of Itolizumab – the murine monoclonal antibody called ior T1 – had therapeutic effects against psoriasis and rheumatoid arthritis.9 Itolizumab, being a humanized version of ior T1, is less immunogenic and has a better side effects profile with the same therapeutic profile when compared to ior T1.9,16
Mechanism of action
Several modes of action have been suggested for Itolizumab; however, many of them need further explanation. Itolizumab binds to CD6, modulating T-lymphocyte activation and proliferation induced by CD6 co-stimulation (Figure 3).2,14,16,18 It did not inhibit soluble ALCAM binding to CD6-expressing HEK293 cells (human embryonic kidney cells)13 and did not cause T-cell depletion in patients treated for rheumatoid arthritis.14,16,20 The various anti-CD6 antibody molecules, such as SPV L14.2 (anti-CD6 IgG1), 161.8 (anti-CD6 IgG1), M-T605 (anti-CD6 IgG1), etc, used for experimental purposes, directly interfered with T-cell proliferation.10,22 Moreover, the presence of CD6 in different types of T-cells and B-cells makes us expect a wider range of immunomodulating effects by Itolizumab, which is not seen clinically.23
Figure 3.
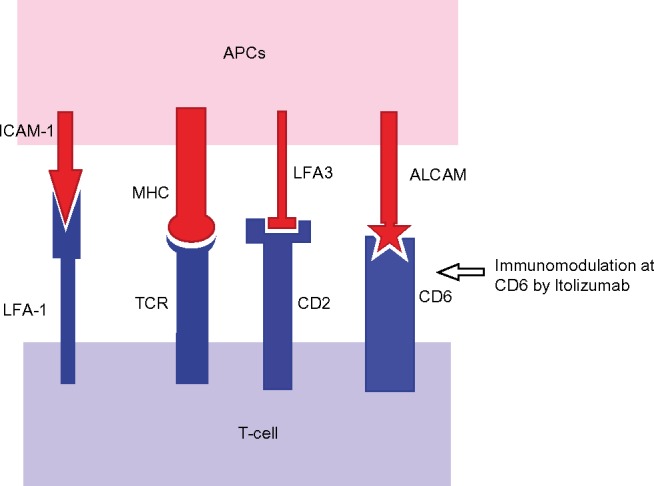
Co-stimulatory signals between APCs and T-cells, and modulation at the CD6 level.
Abbreviations: APCs, antigen-presenting cells; ALCAM, activated leukocyte-cell adhesion molecule; ICAM, inter cellular adhesion molecule; LFA, lymphocyte function associated antigen; MHC, major histocompatibility complex; TCR, T-cell receptor.
Pre-clinical studies have also shown that Itolizumab inhibits intracellular phosphoproteins like mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcriptor3 (STAT-3), which are involved in intracellular signaling pathways triggered by CD6.2,18,20,24 STAT-3 is also important in Th17 development.20 It is also found to down-regulate the gene transcription of pro-inflammatory cytokines and adhesion molecules. This leads to decreased levels of IFN-γ, IL-6, and TNF-α, leading to reduction in the T-cell infiltration at the sites of inflammation.14,20 It does not induce T- or B-cell depletion mediated by CDC (complement-dependent cytotoxicity), ADCC (antibody-dependent cell-mediated cytotoxicity), or apoptosis.14
Pharmacokinetics and dynamics
A linear, dose-dependent relationship was observed in maximum drug concentration levels (Cmax). Increased frequency of administration was associated with increased accumulation of the drug.18 The half-life (T½) of the drug was found to range between 11.72–18.51 days across different dose schedules.25 A single and repeated dose toxicity study done in rats showed that Itolizumab was well tolerated and the no observed adverse effect level (NOAEL) was considered to be 16 mg/kg/day.18
Indications
Indications for Itolizumab are moderate to severe cases of psoriasis.2,25–27 Approval for indications was given by the Central Drug Standard Control Organization, Ministry of Health and Family Welfare, Government of India.
Dosage and administration
The recommended dosage for plaque psoriasis is 1.6 mg/kg body weight, once every 2 weeks for 12 weeks, and 1.6 mg/kg once in 4 weeks until 24 weeks has elapsed.18,26 Itolizumab should be administered as IV infusion made by mixing 250 mL of sterile normal saline after the solution has reached room temperature.18 Slow infusion should be given using an infusion pump by administering approximately 50 mL in the first hour and the remaining 200 mL in the next hour. No other agents should be mixed with the infusion.18
Contraindications and monitoring
The drug is contraindicated in active infections and known hypersensitivity to any of the components of Itolizumab injection or murine proteins.18 Patients should be screened for the presence of all active and latent infections before starting the therapy. It is safer to avoid this drug in patients with neutropenia and lymphopenia, acquired immune deficiency syndrome (AIDS), tuberculosis, and hepatitis B and C, as the effect of Itolizumab has not been evaluated in such conditions.18 Safety of Itolizumab has not been established in pregnant and nursing mothers, children <18 years of age, and in patients with hepatic and renal impairment.18 Itolizumab can cross the placental barrier.18
Adverse effects
Safety data on Itolizumab are derived mostly from the two multicenter clinical trials done on patients with chronic plaque psoriasis and from a study done on rheumatoid arthritis patients.16,25,26 No treatment-related severe adverse effects necessitating discontinuation or reduction of drug dosage were noted in either of the clinical trials.25,26 The various adverse events associated with usage of Itolizumab are compiled in Table 1. The commonest adverse effect encountered was infusion reactions.16,25,26 The frequency and severity of infusion reactions were noted to decrease with subsequent infusions. The symptoms of infusion reaction included nausea, rash, urticaria, flushing, cough, wheezing, dyspnea, dizziness, and headache.26
Table 1.
Adverse effects profile of Itolizumab
| Common adverse effects encountered | Anand et al25 n=40(moderate–severe psoriasis) |
Krupashankar et al26 n=225/52 weeks (moderate–severe psoriasis) |
Rodriguez et al16 n=15/24 weeks (rheumatoid arthritis) |
|---|---|---|---|
| Infusion-related reaction | Chills, 5.69% Pyrexia, 4.88% |
Acute, 17% Delayed, 3.6% Pyrexia, 8.5% |
Headache, 73% Pyrexia, 60% Nausea, 46% |
| Pyrexia due to infections | ND | 1.3% URTI, 7.6% |
UTI, three patients |
| Pruritus | ND | 5.4% | 40% |
| Anti-drug antibody | One patient tested positive | 15.7% no effect on safety and efficacy of drug | Transient and low levels |
| Effect on blood cells | ND | Small and transient decrease in mean absolute lymphocyte count | WBC count and absolute lymphocyte count within normal limits |
Abbreviations: ND, no data; URTI, upper respiratory tract infection; UTI, urinary tract infection; WBC, white blood cell.
The Phase III study26 showed that the undesirable effects were found to be higher in treated group compared to placebo group but this difference was not statistically significant. Of the total 223 patients, 15.2% had at least one episode of acute infusion reaction. Incidence of infections noted with Itolizumab therapy was less compared to placebo group (arm A, 11.1%; arm B, 8.9%; Placebo group, 18.6%). Reactivation of tuberculous lymphadenitis was reported in a patient 4 weeks after Itolizumab therapy, and a single case of septic arthritis occurred in a patient 3 months after the last dose of Itolizumab. The other serious side effects mentioned were erythrodermic psoriasis, exfoliative dermatitis, and adjustment reaction with anxiety noted in single patient.26
The effect of Itolizumab on blood cells also was not dramatic. A Phase III study26 observed a small and transient decrease in mean absolute lymphocyte count, which was not found to be associated with any increase in incidence of infections. The white blood cell and absolute lymphocyte count were within normal limits throughout the study by Rodriguez et al,16 regardless of the dosage. Mild thrombocytosis and anemia were noted in a few patients but could not be fully attributed to the drug treatment, as both conditions can be otherwise associated with rheumatoid arthritis.
Immunogenicity of Itolizumab
One of the major limitations of the use of biologicals is the development of anti-idiotype antibodies leading to decreased serum levels and efficacy of the drug. In a Phase III study26 anti-drug antibody (ADA) presence was assessed, and it was found that 35 (15.75%) patients were positive for ADAs. Out of these, 31 (13.9%) patients had developed ADAs for the first time, and the remaining four (1.79%) patients showed an increase in titers from baseline. However, no clinical correlation between increased ADA titers and adverse events or decreased efficacy could be established. In a Phase II trial,25 immunogenicity analysis was done, and only one patient at week 12 showed positivity, which did not correlate with adverse events and did not cause an altered pharmacokinetic profile. In an exploratory study by Rodriguez et al16 of Itolizumab on rheumatoid arthritis patients, the immunogenicity of the drug was assessed by estimating the IgG anti-idiotype antibody response weekly for 10 weeks after the first dose administration. It was found that the anti-idiotype response was low, transient, and independent of the dose of administered drug. According to Rodriguez et al, the low anti-idiotype antibody response to Itolizumab as the cause for the longer duration of its therapeutic effects compared to its murine predecessor. The complete profiling of side effects requires long duration studies with larger sample size.
Other interactions
As studies examining the interactions of itolizumab with other biologicals are not available to date, the manufacturing company themselves do not recommend its use with other biologicals. The effect of Itolizumab on vaccinations has not been studied.18 As it may affect the efficacy of vaccinations, any live/attenuated vaccines are not recommended in combination with Itolizumab.
Efficacy of Itolizumab in psoriasis
Aira et al2 conducted a study on the immunological and histological effects of Itolizumab on 26 patients who were included in a clinical trial. T-cells and cytokine levels were assessed by comparing the patients’ peripheral blood mononuclear cells (PMBC) to that of healthy donors in vitro. The results suggested that Itolizumab affected the T-cell proliferation capacity and decreased the number of IFN-γ-secreting cells. IL-6, TNF, and IFN-γ showed significant decrease, but IL-10 levels did not change. Histopathological evidence of improvement of lesions in terms of decrease in epidermal thickness and parakeratosis and development of well-defined granular layer were noted after eight administrations of Itolizumab.2
Two randomized multicenter studies were carried out assessing the role of Itolizumab in psoriasis. Both the studies involved patients with stable chronic plaque psoriasis of duration ≥18 years affecting ≥10% of body surface area. The patients had history of either resistance or intolerance to previous systemic therapy.25,26
A Phase II study done by Anand et al25 was a randomized, single-blind study that had 40 patients randomized to eight groups of five patients each. They were given Itolizumab at different doses at different intervals as IV injections. The total treatment period was 8 weeks, with a follow-up period of up to 24 weeks. In the overall cohort study (n=40), statistically significant improvement in mean PASI score (psoriasis area severity index score), physician’s global assessment (PGA) score, and psoriasis severity scale (PSS) were noted. The best therapeutic response was observed in the cohort receiving a dose of 1.6 mg/kg once every 2 weeks. The DLQI (dermatology life quality index) and SF-6 (short form-6) estimates showed improvement in the quality of life of patients.25
The second study was a 52-week Phase III, double-blind, placebo-controlled, randomized, parallel-arm, one-way crossover multicenter trial.26 The study included 225 patients with moderate to severe psoriasis with PASI score ≥10. They were randomized 2:2:1 to three groups A, B, and C. From 0–12 weeks, groups A and B were assigned different dosing schedules of Itolizumab, and C was kept as placebo. After 12 weeks, placebo crossover was done and the drug was given to group C. The efficacy of treatment (PASI score and PGA), quality of life (SF-6 and DLQI), histopathological improvement, and safety features including ADA response were assessed. At week 12, statistically significant improvement in PASI scores was noted in the treatment arms compared to placebo group. After crossover of the placebo group to receive Itolizumab at 12 weeks, rapid improvement was noted, and the PASI scores were comparable to the other treatment arms by week 20. PGA and quality scores showed similar trends as PASI scores. Incidence of adverse effects over 52 weeks was similar in patients assigned to any of the treatment arms or the placebo group. The efficacy data of both Phase II and Phase III are summarized in Table 2.
Table 2.
Efficacy of Itolizumab in moderate to severe psoriasis cases
| Anand et al25 Phase II clinical trial |
Krupashankar et al26 Phase III clinical trial |
|
|---|---|---|
| Mean PASI score at baseline | 22.32±8.84 | 21.3±8.46 |
| PASI-75 score at 12 weeks | 45% | Arm A, 27% (A vs C, P=0.0172) Arm B, 36.4% (B vs C, P=0.043) Arm C (placebo), 2.3% |
| PGA score at 12 weeks (clear or minimal) | 65% | A – 20% (A vs C, P<0.0001) B – 16% (B vs C, P<0.0002) C – 4.9% |
| Mean change from baseline PGA score at 12 weeks | P<0.0001 | A vs C, P<0.0001 B vs C, P=0.0002 |
| Mean change from baseline short form-36 at week 12 | Improvement noted | A – 3.5±6.5 B – 2.4±6.8 C – 1.7±6.8 |
| DLQI score at 12 weeks | Improvement noted | A – 7.3±6.6 B – 5.8±5.8 C – 9.0±7.9 |
| Reduction from baseline in mean epidermal thickness at 12 weeks | P=0.0005 | Improvement noted |
| Reduction from baseline in mean rate thickness at 12 weeks | P<0.0001 | Improvement noted |
Abbreviations: PASI, psoriasis area severity index; PGA, physician’s global assessment; DLQI, dermatology life quality index; vs, versus.
Comparison with other biologicals used for psoriasis
Being a relatively new drug, no comparative studies for Itolizumab are available at present. Comparisons made from available data show that the incidence of infections reported for Itolizumab (18%)26 was lower when compared to other biological agents. With regard to the efficacy, the PASI-75 response of Infliximab, Adalimumab, Etanercept,28 and Ustekinumab were found to be higher when compared to that of Itolizumab. The comparison of different biologicals with Itolizumab is given in Table 3.
Table 3.
Comparison of Itolizumab with different biologicals used for treating psoriasis
| Drug | Study | N/dur in weeks | PASI-75, 12 weeks | Anti-drug antibodies | Incidence of infections |
|---|---|---|---|---|---|
| Alefacept | Ellis et al33 | 229/24 | 0.025 mg/kg, 33% 0.05 mg/kg, 31% |
One patient | |
| Lebwohl et al34 (Phase III RDBPC) | 507/24 | 15 mg, 33% 10 mg, 28% |
4% | 14%–16% | |
| Adalimumab | Gordon et al35 (Phase II RDBPC) | 147/60 | – | Not reported | – |
| Menter et al30 (Phase III RDBPC) | 1,212/52 | Alternate weeks therapy, 53% Weekly, 80% |
8.8% associated decreased response | 29% | |
| Gottileb et al36 (Phase II RDBPC) | 112/24 | 30% | Not reported | URTI, 35% Sinusitis,14% |
|
| Etanercept | Tyring et al37 (Phase III RDBPC) | 618/96 | 47.7% | 18.3% No effect on safety and efficacy |
103.9 events/100 patient years |
| Leonardi et al38 (Extension of Phase III trial RDBPC) | 652/24 | 25 mg biweekly, 34% 50 mg biweekly, 49% |
Eight patients | URTI, 12%–14% Sinusitis, %–6% |
|
| Infliximab | Gottlieb et al39 (Phase II RDBPC trial) | 249/30 | Week 10: 3 mg/kg, 72% 5 mg/kg, 88% |
3 mg/kg, 27% 5 mg/kg, 20% |
– |
| Menter et al40 (Phase III RDBPC trial) | 835/50 | Week 10: 3 mg/kg, 70% 5 mg/kg, 76% |
3 mg/kg, 49% and 5 mg/kg, 39% associated decreased response |
– | |
| Reich et al29 (Phase III RDBPC) | 378/72 | Week 10, 80% | 27% associated decreased response | 42% | |
| Ustekinumab | Papp et al41 (Phase III RDBPC) | 1,230/52 | 45 mg, 66.7% 90 mg, 75.7% |
12.7% | 31% |
| Leonardi et al31 (Phase III RDBPC) | 766/72 | 45 mg, 66.1% 90 mg, 66.4% |
5.1% | 21% | |
| Itolizumab | Anand et al25 (Phase II RSB trial) | 40/32 | 45% | One patient tested positive no effect on safety and efficacy | – |
| Krupashankar et al26 (Phase III RDBPC) | 225/52 | Arm A 27% Arm B 36.4% Placebo 2.3% |
15.7% no effect on safety and efficacy | 18% |
Abbreviations: N, number of patients; dur, duration of study; RDBPC, randomized, double-blind, placebo-controlled; RSB, randomized, single-blind; PASI, psoriasis area severity index; URTI, upper respiratory tract infection; –, data is not clearly mentioned.
Other uses of Itolizumab
Itolizumab has been proposed to be effective in autoimmune diseases such as rheumatoid arthritis, Sjogren’s syndrome, and multiple sclerosis. Increased expression of CD6 ligand ALCAM is reported in salivary gland epithelial cells in Sjogren’s syndrome, synovium of rheumatoid arthritis patients, and blood–brain barrier endothelium of multiple sclerosis patients.19 In Sjogren’s syndrome, it has been found that the CD6 ligand ALCAM is over-expressed and that CD6-positive T- and B-cells are detected within salivary glands of affected individuals.19
Genome-wide association scans have identified the susceptibility foci in regions of genes with immune function including CD6 in patients with multiple sclerosis.32 An open-label, non-controlled Phase I trial by Rodriguez et al,16 done on 15 rheumatoid arthritis patients, showed good therapeutic response that lasted for 4 weeks after the last dose of Itolizumab and with a safer side effect profile. A preliminary study21 assessing the therapeutic role of Itolizumab in B-cell chronic lymphocytic leukemia and cutaneous T-cell lymphoma show promising results; however, larger studies are required to make any definitive conclusions.
To conclude, Itolizumab seems to be a safer option for moderate to severe psoriasis when compared to other biologicals indicated for the same type of psoriasis, as it does not inhibit or directly deplete T-cells. The development of ADAs is also less pronounced with Itolizumab. However, its efficacy seems to be less when compared to other biologicals, based on the available literature. Only a single comparison study, which is randomized, double-blind, and placebo-controlled, is available.26 Larger studies involving bigger sample sizes and longer duration are required to fully characterize the therapeutic and safety profile of the drug. Furthermore, long-term animal studies have not been conducted with Itolizumab to evaluate its carcinogenic or teratogenic potential. In addition, comparison studies with other biological agents are also warranted.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kandpur S, Bhari N. Newer targeted therapies in psoriasis. Indian J Dermatol Venereol Leprol. 2013;79(Suppl 7):S47–S52. doi: 10.4103/0378-6323.115532. [DOI] [PubMed] [Google Scholar]
- 2.Aira LE, López-Requena A, Fuentes D, et al. Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab. MAbs. 2014;6(3):783–793. doi: 10.4161/mabs.28376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahajan R, Handa S. Pathophysiology of psoriasis. Indian J Dermatol Venereol Leprol. 2013;79(Suppl 7):S1–S9. doi: 10.4103/0378-6323.115505. [DOI] [PubMed] [Google Scholar]
- 4.Raychaudhuri SP, Nguyen CT, Raychaudhuri SK, Gershwin ME. Incidence and nature of infectious disease in patients treated with Anti-TNF agents. Autoimmun Rev. 2009;9(2):67–81. doi: 10.1016/j.autrev.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Strangfeld A, Listing J. Infections and musculoskeletal conditions: bacterial and opportunistic infections during anti-TNF therapy. Best Pract Res Clin Rheumatol. 2006;20(6):1181–1195. doi: 10.1016/j.berh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Pallavicini FB, Caporali R, Sarzi-Puttini P, et al. Tumour necrosis factor antagonist therapy and cancer development: analysis of the LORHEN registry. Autoimmun Rev. 2010;9(3):175–180. doi: 10.1016/j.autrev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The role of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol. 2012;51(4):389–395. doi: 10.1111/j.1365-4632.2011.05154.x. [DOI] [PubMed] [Google Scholar]
- 8.Stockinger B, Veldhoen M. Differentiation and function of Th17T cells. Curr Opin Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Ramirez R, Loisel S, Buors C, et al. Rationale for targeting CD6 as a treatment for autoimmune diseases. Arthritis. 2011 Feb 10; doi: 10.1155/2010/130646. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimferrer I, Calvo M, Mittelbrunn M, et al. Relevance of CD 6-mediated interactions in T cell activation and proliferation. J Immunol. 2004;173:2262–2270. doi: 10.4049/jimmunol.173.4.2262. [DOI] [PubMed] [Google Scholar]
- 11.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Eng J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 12.Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4(3):321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 13.Alonso R, Huerta V, de Leon J, et al. Towards the definition of a chimpanzee and human conserved CD6 domain 1 epitope recognized by T1 monoclonal antibody. Hybridoma (Larchmt) 2008;27(4):291–301. doi: 10.1089/hyb.2008.0007. [DOI] [PubMed] [Google Scholar]
- 14.Beck A, Wurch T, Reichert JM. 6th Annual European Antibody Congress 2010: November 29–December 1, 2010, Geneva, Switzerland. MAbs. 2011;3(2):111–132. doi: 10.4161/mabs.3.2.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer NG, Fox DA, Haqqi TM, et al. CD6: expression during development, apoptosis and selection of human and mouse thymocytes. Int Immunol. 2002;14(6):585–597. doi: 10.1093/intimm/dxf025. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez PC, Torres-Moya R, Reyes G, et al. A clinical exploratory study with itolizumab, an anti-CD6 antibody, in patients with rheumatoid arthritis. Results Immunol. 2012;2:204–211. doi: 10.1016/j.rinim.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen MA, Patel DD, Li X, et al. Cloning, mapping and characterization of activated leucocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med. 1995;181:2213–2220. doi: 10.1084/jem.181.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzumab™ (Itolizumab) solution for iv infusion [prescribing information] India: Biocon, Inc; 2013. [Accessed April 3, 2015]. Available from: http://www.biocon.com/docs/prescribing_information/immunotherapy/alzumab_pi.pdf. [Google Scholar]
- 19.Le Dantec C, Alonso R, Fali T, et al. Rationale for treating primary Sjögren’s syndrome patients with an anti-CD6 monoclonal antibody (Itolizumab) Immunol Res. 2013;56(2–3):341–347. doi: 10.1007/s12026-013-8423-x. [DOI] [PubMed] [Google Scholar]
- 20.Nair P, Melarkode R, Rajkumar D, Montero E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol. 2010;162:116–130. doi: 10.1111/j.1365-2249.2010.04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izquierdo-Cano L, Espinosa-Estrada E, Hernández-Padrón C, et al. Anticuerpo monoclonal humanizado itolizumab (anti-cd6) en síndromes linfoproliferativos cd 6+. Experiencia preliminar. [Humanized monoclonal antibody Itolizumab (anti-cd6) in patients with lymphopro-liferative disorders cd6 +. Preliminary experience] Revista Cubana de Hematología, Inmunología y Hemoterapia. 2014;30(3):0–0. Spanish. [Google Scholar]
- 22.Gimferrer I, Farnós M, Calvo M, et al. The accessory molecules CD5 and CD6 associate on membrane of lymphoid T cells. J Biol Chem. 2003;278(10):8564–8571. doi: 10.1074/jbc.M209591200. [DOI] [PubMed] [Google Scholar]
- 23.Jayaraman K. Biocon’s first-in-class anti-CD6 mAb reaches the market. Nat Biotechnol. 2013;31(12):1062–1063. doi: 10.1038/nbt1213-1062b. [DOI] [PubMed] [Google Scholar]
- 24.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 25.Anand A, Assudani D, Nair P, et al. Safety, efficacy and pharmacokinetics of T1h, a humanized anti-CD6 monoclonal antibody, in moderate to severe chronic plaque psoriasis – results from a randomized phase II trial. J Immunol. 2010;184:96–13. abstract. [Google Scholar]
- 26.Krupashankar DS, Dogra S, Kura M, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol. 2014;71(3):484–492. doi: 10.1016/j.jaad.2014.01.897. [DOI] [PubMed] [Google Scholar]
- 27.Reichert JM. Which are the antibodies to watch in 2013? MAbs. 2013;5(1):1–4. doi: 10.4161/mabs.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EnbrelR (Etanercept) [ package insert] US: Amgen; 1997. [Accessed April 3, 2015]. Available from: http://pi.amgen.com/united_states/enbrel/derm/enbrel_pi.pdf. [Google Scholar]
- 29.Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 30.Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2004;51:534–542. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Leonardi CL, Kimball AB, Papp KA, et al. PHOENIX 1 study investigators Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 32.Kofler DM, Severson CA, Mousissian N, De Jager PL, Hafler DA. The CD6 multiple sclerosis susceptibility allele is associated with alterations in CD4+ T cell proliferation. J Immunol. 2011;187:3286–3291. doi: 10.4049/jimmunol.1100626. [DOI] [PubMed] [Google Scholar]
- 33.Ellis CN, Krueger GG, Alefacept Clinical Study Group Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345(4):248–255. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 34.Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE, Alefacept Clinical Study Group An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139(6):719–727. doi: 10.1001/archderm.139.6.719. [DOI] [PubMed] [Google Scholar]
- 35.Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb AB, Matheson RT, Lowe N, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Tyring S, Gordon KB, Poulin Y, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Arch Dermatol. 2007;143(6):719–726. doi: 10.1001/archderm.143.6.719. [DOI] [PubMed] [Google Scholar]
- 38.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept Psoriasis Study Group Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51(4):534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31, e1–e15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Papp KA, Langley RG, Lebwohl M, et al. PHOENIX 2 study investigators Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]


