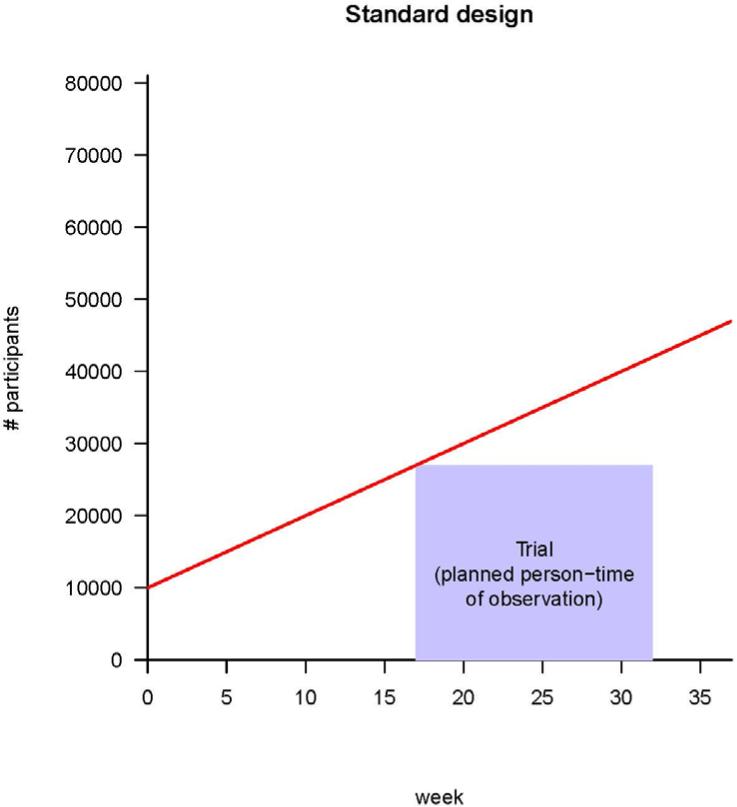
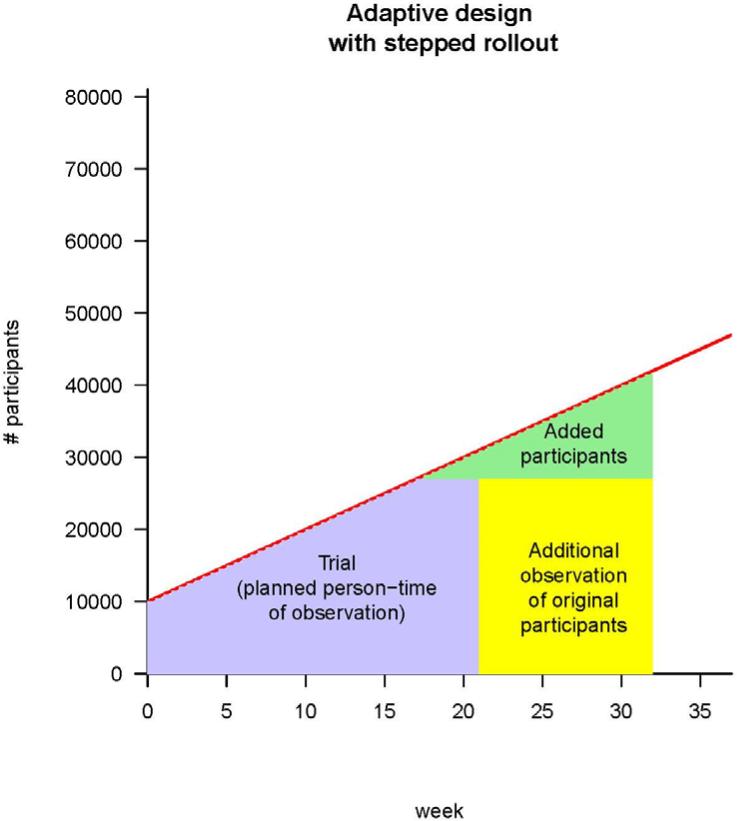
Fig. 1.
Strategies for vaccine trials in challenging conditions. (A) A classic iRCT. Randomization has occurred within the whole population, and the trial can only start when all of the centers are ready. The red line represents the maximum number of participants the trial could enroll, given increasing logistical capacity. The area of the box is the total person-time in the trial; it is the size of the trial measured in person-weeks of observation. (B) Illustration of stepped rollout (beginning the trial at each center when that center becomes ready to participate) and adaptive design [planning to add persons in new centers (green) and/or extend person-time of observation in existing centers (yellow), depending on incidence]. The estimated person-time required to do the trial (purple box) is the same as in (A), but because under stepped rollout the trial can start earlier, it can end earlier. Parameters are all illustrative, and (for simplicity) centers are shown as be-coming ready in a linear manner with time.