Abstract
Background
Patients who have suffered from cerebral ischemia have a high risk of recurrent vascular events. Predictive models based on classical risk factors typically have limited prognostic value. Given that cerebral ischemia has a heritable component, genetic information might improve performance of these risk models. Our aim was to develop and compare two models: one containing traditional vascular risk factors, the other also including genetic information.
Methods and Results
We studied 1020 patients with cerebral ischemia and genotyped them with the Illumina Immunochip. Median follow-up time was 6.5 years; the annual incidence of new ischemic events (primary outcome, n=198) was 3.0%. The prognostic model based on classical vascular risk factors had an area under the receiver operating characteristics curve (AUC-ROC) of 0.65 (95% confidence interval 0.61-0.69). When we added a genetic risk score based on prioritized SNPs from a genome-wide association study of ischemic stroke (using summary statistics from the METASTROKE study which included 12389 cases and 62004 controls), the AUC-ROC remained the same. Similar results were found for the secondary outcome ischemic stroke.
Conclusions
We found no additional value of genetic information in a prognostic model for the risk of ischemic events in patients with cerebral ischemia of arterial origin. This is consistent with a complex, polygenic architecture, where many genes of weak effect likely act in concert to influence the heritable risk of an individual to develop (recurrent) vascular events. At present, genetic information cannot help clinicians to distinguish patients at high risk for recurrent vascular events.
Introduction
Patients who suffered from cerebral ischemia have an increased (long-term) risk of new cerebrovascular and cardiovascular events. The American Heart Association recommends the Framingham risk score as a prediction model for major vascular events.[1, 2] Recently this model was compared with six other models in terms of calibration and discrimination.[3] Almost all models slightly overestimated the risk for major events in low and high risk patients. Addition of genetic information might improve these models, but so far this has not yet been evaluated.
Several studies have been performed with a candidate gene approach to find an association between single nucleotide polymorphisms (SNPs) and first ischemic strokes, with often conflicting results, possibly due to small sample sizes in earlier studies and heterogeneity of the stroke subtypes. Two loci (PITX2 and ZFHX3) were found to be associated with atrial fibrillation and cardioembolic stroke risk.[4, 5] Subsequently, two loci (9p21 and HDAC9) were identified as robust associations with large vessel stroke.[6, 7] These findings were recently confirmed in a meta-analysis of genome-wide association studies (GWAS) carried out by the METASTROKE consortium.[8] These common genetic polymorphisms account only for a small increase in disease risk, suggesting that large sample sizes will be needed to find additional susceptibility alleles.[9]
Associations between genetic polymorphisms and recurrence of vascular events following an initial episode of cerebral ischemia have received little attention. The aim of our study was to assess the additional value of genetic information in prognostic models in a hospital based cohort of patients who have suffered from cerebral ischemia of arterial origin (CIAO). We benefited from continuing efforts in the international stroke genetics community by incorporating results from the METASTROKE study.[8] With the observed effect estimates for selected SNPs (enriched for association with ischemic stroke), we calculated a genotype-based score for each individual in our cohort. Because the data from the GWAS were collected independently from our cohort, we were able to directly and rigorously assess the prognostic value of our prediction models.
Methods
Study design and patient population
The rationale of this study is described elsewhere in detail.[10] We collected data of patients with non-disabling cerebral ischemia of arterial origin, who were referred to the University Medical Center Utrecht, The Netherlands and were included in the SMART (Second Manifestations of Arterial disease) study, or the Utrecht Stroke Database (USDB). A detailed description of the SMART study was published previously.[11] Briefly, patients who gave their written informed consent underwent a standardised vascular screening programme, including a health questionnaire, laboratory assessment, and ultrasonography to investigate the prevalence of additional vascular diseases. Patients were followed up with bi-annual questionnaires. In the USDB extensive baseline data have been collected for consecutive patients visiting the University Medical Center Utrecht for TIA or stroke since 1991. Patient data was used anonymized in our analyses. Blood samples were taken from 1999 onwards and stored in the Neurology Blood Bank. Follow-up data were collected by contacting these patients or their general practitioners. Patients with non-atherosclerotic causes of cerebral ischemia or with potential source of embolism in the heart were excluded from this study. We therefore included only patients with cerebral ischemia of arterial origin. For the current study, the data of 1125 patients were available. These patients were included between April 1994 and May 2009.
Outcomes
The primary outcome event was defined as a composite of the first occurrence of myocardial infarction, ischemic stroke or vascular death not due to hemorrhage. Secondary outcome event was recurrent ischemic stroke (Table 1). For potential outcome events reported by the patient we retrieved hospital discharge letters and the results of relevant laboratory and radiology examinations. Three members of the SMART Outcome Committee independently audited events on basis of available information. This committee consisted of physicians from different departments. In case of disagreement, consensus was reached by consulting other members of the Outcome Committee. Potential outcomes in patients included from the USDB were audited similarly.
Table 1. Definitions of outcome events.
| Event | Definition |
|---|---|
| Ischemic stroke | Relevant clinical features that caused an increase in impairment of at least one grade on the modified Rankin scale21 associated with a relevant infarction on a repeat brain scan. |
| Myocardial infarction | At least two of the following criteria: |
| 1: Chest pain >20 min, not disappearing after administration of nitrates | |
| 2: ST elevation >1 mm in two following leads or a left bundle branch block | |
| 3:CK elevation of at least two times its normal value and an MB fraction >5% of total CK | |
| Vascular death: not due to hemorrhage | Sudden death: unexpected coronary death occurring within 1 h after onset of symptoms or within 24 h given convincing circumstantial evidence. |
| Terminal heart failure | |
| Fatal myocardial infarction or ischemic stroke |
Primary outcome was defined as all fatal and non-fatal ischemic events. Secondary outcome was ischemic stroke separately.
Genotyping
DNA samples available from both studies and stored in a -70 degrees Celsius freezer were transported to the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, Rotterdam, The Netherlands, where the samples were genotyped with the Illumina Immunochip.[12] The goal of the Immunochip was to provide a cost-effective genotyping platform for deep follow-up replication studies. It includes about 200,000 SNPs, selected in part on the basis of association results (low p-values) from a wide range of GWAS of immune-related diseases as well as the diseases covered by the second round of the Wellcome Trust Case Control Consortium (WTCCC-2), including ischemic stroke (http://www.wtccc.org.uk/ccc2/wtccc2_studies.shtml). About 2500 SNPs from an early analysis of the ischemic stroke GWAS by WTCCC-2 were contributed to the Immunochip design. We used these specific SNPs for our further analyses.
Quality control steps consisted of filtering of SNPs and individuals with >5% missing data, followed by filtering of SNPs with a minor allele frequency (MAF) <1% or deviation from Hardy-Weinberg equilibrium (HWE; p < 10−6). We then used individual-pairwise identity-by-state estimates to remove (unknown) related and potentially contaminated samples. We pruned SNPs by their pairwise linkage disequilibrium to arrive at a set of independent SNPs. Data processing and quality control filtering were performed in PLINK.[13] Principal components analysis was used to check genetic clustering of all individuals against reference individuals from the HapMap.[14]
Individual genetic risk score
For each individual patient we calculated a genetic risk score on the basis of the genotypes of 1501 QC-passing SNPs and their effect sizes that were independently obtained from the GWAS of ischemic stroke led by the METASTROKE consortium.[8] Using PLINK, we calculated the genetic risk score as the sum of the ln(OR) multiplied by the number of risk alleles carried for each SNP considered in a given individual, following a method described elsewhere. [15]
Statistical analysis
The prediction models were built with Cox regression analyses in SPSS. In univariable analysis we calculated the hazard ratios and corresponding 95% confidence intervals (CI) of different stroke risk factors. The different clinical variables included were adapted from a previous study, in which they determined long term survival and recurrent vascular event risk in patients with cerebral ischemia.[16] We then constructed the prediction model, in which we sequentially entered variables from the patients’ history until no remaining candidate variable had a significance level of <0.10 and into which we forced the genetic risk score based on METASTROKE effect estimates. Next we constructed receiver-operator characteristics (ROC) curves to compare the discriminatory performance with its area under the curve (AUC) of the model with and without genetic information. All analyses were done for the primary and secondary outcome.
Results
Baseline
Data were available of 1125 patients presenting with transient or non-disabling manifestations of cerebral or retinal ischemia and with information on genetic variants. Of these, 105 patients had to be excluded from further analysis because of genotyping quality concerns or unexpected relatedness between patients. These patients were similar to the remaining patients with respect to baseline characteristics (Table 2).
Table 2. Baseline characteristics.
| Included patients | Excluded patients | ||
|---|---|---|---|
| 1020 | 105 | ||
| Patients with primary outcome | Patients without primary outcome | ||
| 198 | 822 | ||
| Age (years) (mean, SD) | 66 (10) | 62 (11) | 63 (11) |
| Male sex | 150 (76%) | 518 (63%) | 68 (65%) |
| Qualifying diagnosis | |||
| TIA | 75 (38%) | 283 (34%) | 30 (28%) |
| Stroke | 93 (47%) | 451 (55%) | 66 (62%) |
| Transient Monocular Blindness | 24 (12%) | 79 (10%) | 6 (6%) |
| Retinal infarction | 6 (3%) | 9 (1%) | 3 (3%) |
| Subtype Qualifying diagnosis | |||
| Large vessel disease | 141 (71%) | 552 (67%) | 57 (54%) |
| Small vessel disease | 57 (29%) | 270 (33%) | 48 (46%) |
| History | |||
| Stroke | 53 (27%) | 161 (20%) | 17 (16%) |
| Carotid surgery | 14 (7%) | 21 (3%) | 3 (3%) |
| Myocardial infarction | 37(19%) | 80 (10%) | 9 (9%) |
| Vascular surgery | 51 (26%) | 119 (15%) | 16 (15%) |
| Hypertension | 99 (50%) | 391 (48%) | 50 (47%) |
| Diabetes Mellitus | 37 (19%) | 106 (13%) | 19 (18%) |
| Hyperlipidemia | 60 (30%) | 280 (34%) | 36 (34%) |
| Cigarette smoking | |||
| Currently | 35 (18%) | 198 (24%) | 28 (26%) |
| Never or ever | 159 (81%) | 559 (73%) | 71 (67%) |
| Blood pressure (mm Hg) | |||
| Systolic (mean, SD) | 157 (28) | 149 (25) | 153 (28) |
| Diastolic (mean, SD) | 85 (14) | 84 (13) | 84 (14) |
| Glucose (mmol/L) (mean, SD) | 6.6 (2.4) | 6.4 (1.9) | 6.5 (2.0) |
* Patients were excluded because of quality concerns
At baseline patients had a mean age of 63 years and 66% was male (Table 2). The index event was a minor stroke in 53% of the patients, 35% had had a TIA and 12% suffered from an ischemic ocular event. Almost 50% of the patients had hypertension and 20% suffered from an earlier stroke. Patients with a recurrent ischemic vascular event had overall more risk factors for vascular disease than patients who had no recurrence.
Follow up
The median follow-up time was 6.5 years (6630 person-years). The follow-up was complete in 99.5% of the patients. The annual risk of ischemic events was 3.0% (198 events). Half of the events were a fatal or non-fatal ischemic stroke (98 patients), 65 events concerned fatal or non-fatal myocardial infarction and the remaining 35 were other vascular death.
Prognostic value of classical vascular risk factors
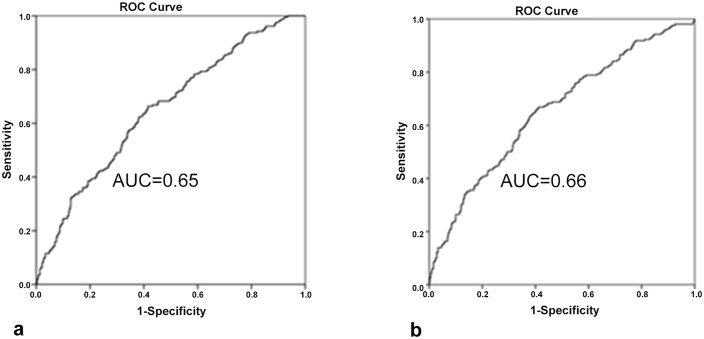
The different AUC-ROC values for the primary and secondary outcomes are displayed in Table 3 and for the primary outcome the ROC-curves are displayed in Fig 1. For the primary outcome the prognostic model consisted of the variables age, sex, myocardial infarction, intermittent claudication, diabetes mellitus and vascular surgery. The AUC-ROC was 0.65 (95% CI 0.61–0.69). For the secondary outcome, the AUC-ROC was 0.60 (95% CI 0.54–0.66), this included the vascular risk factors age, myocardial infarction, and hypertension.
Table 3. Cox proportional hazard models and AUC-ROC.
| M1 | M2 | |||
|---|---|---|---|---|
| Indicator | HR | 95% CI | HR | 95% CI |
| Demographic characteristics | ||||
| Male | 1.47 | 1.06–2.04 | 1.35 | 0.86–2.11 |
| Age | 1.05 | 1.03–1.06 | 1.02 | 1.00–1.04 |
| History | ||||
| Myocardial infarction | 1.78 | 1.25–2.55 | 1.72 | 1.03–2.87 |
| Hypertension | 1.08 | 0.82–1.43 | 1.56 | 1.05–2.33 |
| Intermittent Claudication | 1.76 | 1.14–2.72 | 1.55 | 0.80–2.97 |
| Diabetes Mellitus | 1.37 | 0.96–1.97 | 1.34 | 0.81–2.24 |
| Vascular surgery | 1.79 | 1.30–2.46 | 1.50 | 0.93–2.40 |
| Genetic risk score | 1.13 | 0.92–1.38 | 1.17 | 0.91–1.50 |
| AUC-ROC | ||||
| Only classical risk factors | 0.65 | 0.61–0.69 | 0.60 | 0.54–0.65 |
| Plus genetic risk core | 0.66 | 0.61–0.70 | 0.60 | 0.54–0.66 |
Table displays univariable analyses of risk factors for vascular disease for different endpoints. The bold numbers are accounted in the multivariable model with and without genetic risk scores. AUC-ROC = Area Under Curve of the Receiver Operating Characteristics Curve. M1 = primary outcome. M2 = secondary outcome, ischemic stroke
Fig 1. ROC curves for the primary outcome.
(a) based on classical risk factors only; (b) based on classical risk factors plus the genetic risk score.
Prognostic value of classical risk factors and genetic risk score
The vascular risk factors used in these models remained the same as the ones used in the first model. After addition of the genetic risk score the AUC-ROC for the primary outcome was essentially identical to that of the model with only classical vascular risk factors. For cerebral ischemia the AUC-ROC showed similar patterns (Table 3).
Discussion
In our cohort of patients with cerebral ischemia of arterial origin we found no additional value of genetic information in the prediction of new ischemic events including ischaemic stroke. The overall prognostic performance of the known classical risk factors for vascular diseases is poor (AUC-ROC 0.65; 95%CI 0.61–0.69), consistent with similar prediction models described in the literature.[3] The inclusion of a genetic risk score based on SNPs that could be associated with ischemic stroke did not result in a prognostic model that improved risk stratification.
As far as we know, our study is the first to characterize the prognostic value of genetic information in the assessment of future risk for vascular events after cerebral ischaemia. Our cohort comprises well phenotyped ischemic stroke patients from a single medical center with uniform follow-up data. The origin from a single hospital could also be seen as a limitation with respect to the generalisability of the results. However, we feel that our cohort is representative since the results for classical risk factors for atherosclerosis affecting prognosis, like previous stroke, diabetes mellitus or hypertension, are consistent with other studies.
The sample size is a major limitation. We had to drop almost 10% of our population due to quality concerns of the genetic data. These patients, however, had similar clinical characteristics as the remaining patients. Furthermore we included only non-disabled patients on the (predefined) basis that this patient group is the one most relevant, with the highest chance of survival and long-term prospects for recovery, and therefore represents the main focus of our study.
The selection of SNPs and the nature of the external training data set could be seen as a limitation of our study. We were not aware of another dataset on patients with cerebral ischemia of arterial origin followed prospectively for the occurrence of ischemic events or bleedings in whom also genetic data were available (which we could have used as an alternative training data set). We therefore decided to use the observed effect estimates from the METASTROKE study, even though this study focused on prevalent (ischemic) stroke cases in the population instead of the occurrence of recurrent vascular events. It is certainly possible that genes influencing the risk of a first cerebral ischemic event differ from those genes associated with long-term prognosis after an ischemic event. In addition, we only used the effect estimates for all types of ischemic stroke instead of those for small and large vessel stroke subtypes, because our sample was too small to look into individual subtypes of stroke.
Our results illustrate the complexity of finding susceptibility alleles for a clinical phenotype that is so complex and heterogeneous such as ischemic stroke. To date, only a handful of robust SNP associations have been identified for cardioembolic stroke (PITX2, ZFHX3), large-artery atherosclerotic stroke (9p21, HDAC9), and more recently, for all subtypes of stroke (12q24). [17–18]The approach to test the collective effects of many common variants simultaneously has been successfully applied in complex traits such as schizophrenia and bipolar disorder [15]
We were only informed about the use of medication at baseline of the patients in this study. Platelet function, response to pharmacotherapy and genetic factors may be associated with outcome and recurrent risk. The debate about the role of aspirin resistance and the way patient tailored treatment strategies could be implemented in daily practice is still ongoing.[19–21] The same is true for other antithrombotic treatment strategies as, for example clopidogrel, for which genetic association with its metabolism is reasonably well understood.[22]
Future stroke genetic studies might result in better risk prediction for different stroke subtypes and recurrent events. It remains unresolved today, however, to what extent genetic variants of modest effect (even if many more are identified as a result of larger discovery efforts) could contribute to prognostic models for such complex phenotypes as stroke above and beyond family history and traditional risk factors.
Integrity of research and reporting
The Ethics Committee of the hospital approved both studies (SMART and USDB) from which we used the data. All patients included in these studies gave written informed consent prior to inclusion.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written. The authors declare that they have no conflict of interest.
The data of this study will be made available upon request
Acknowledgments
We would like to thank J. van Setten for her support in the genetic analysis.
SMART study group
A. Algra, MD, Y. van der Graaf, MD, D.E. Grobbee, MD, and G.E.H.M. Rutten, MD, Julius Center for Health Sciences and Primary Care; F.L.J. Visseren, MD, Department of Vascular Medicine; F.L. Moll, MD, Department of Vascular Surgery; L.J. Kappelle, MD, Department of Neurology; W.P.T.M. Mali, MD, Department of Radiology; and P.A. Doevendans, MD, Department of Cardiology, University Medical Center, Utrecht, the Netherlands
METASTROKE consortium
Matthew Traylor, a Martin Farrall, b , c Elizabeth G Holliday, d Cathie Sudlow, e Jemma C Hopewell, f Yu-Ching Cheng, g Myriam Fornage, h M Arfan Ikram, i , j , k Rainer Malik, l Steve Bevan, a Unnur Thorsteinsdottir, m , n Anita L DeStefano, o Bradford B Worrall, p , q Alex P Reiner, r , Wellcome Trust Case Control Consortium 2 (WTCCC2), Braxton D Mitchell, g Robert Clarke, f Christopher Levi, s Sudha Seshadri, t Giorgio B Boncoraglio, u Pankaj Sharma, v Joshua C Bis, r Solveig Gretarsdottir, m Bruce M Psaty,w Peter M Rothwell, x Jonathan Rosand, y , z , aa James F Meschia, ab Kari Stefansson, m , n Martin Dichgans, l Hugh S Markus, a
Stroke and Dementia Research Centre, St George's University of London, London, UK
Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, UK
Department of Cardiovascular Medicine, University of Oxford, Oxford, UK
Center for Clinical Epidemiology and Biostatistics, School of Medicine and Public Health, University of Newcastle, and Center for Bioinformatics, Biomarker Discovery and Information-Based Medicine, Hunter Medical Research Institute, NSW, Australia
Division of Clinical Neurosciences and Insititute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK
Clinical Trial Service Unit and Epidemiological Studies Unit, University of Oxford, Oxford, UK
University of Maryland School of Medicine, Department of Medicine, Baltimore, MD, USA
University of Texas Health Science Center at Houston, Houston, TX, USA
Department of Epidemiology, Erasmus MC University Medical Center, Rotterdam, Netherlands
Department of Neurology and Department of Radiology, Erasmus MC University Medical Center, Rotterdam, Netherlands
Netherlands Consortium for Healthy Ageing, Leiden, Netherlands
Institute for Stroke and Dementia Research, Klinikum der Universitát München, Ludwig-Maximilians-Universität, and Munich Cluster for Systems Neurology (SyNergy), Munich, Germany
deCODE Genetics, Reykjavik, Iceland
Faculty of Medicine, University of Iceland, Reykjavik, Iceland
Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA
Department of Neurology, University of Virginia, Charlottesville, VA, USA
Department of Public Health Science, University of Virginia, Charlottesville, VA, USA
Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Centre for Translational Neuroscience and Mental Health Research, University of Newcastle, and Hunter Medical Research Institute, New Lambton, NSW, Australia
Department of Neurology, Boston University School of Medicine, Boston, MA, USA
Department of Cereberovascular Disease, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Neurologico Carlo Besta, Milan, Italy
Imperial College Cerebrovascular Research Unit (ICCRU), Imperial College London, London, UK
Stroke Prevention Research Unit, Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, UK
Program in Medical and Population Genetics, Broad Institute of Harvard and MIT, Cambridge, MA, USA
Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA, USA
Department of Neurology, Mayo Clinic, Jacksonville, FL, USA
Data Availability
All relevant data are within the paper.
Funding Statement
S. Achterberg is supported in part by a grant from the Netherlands Heart Foundation, (grant No. 2005B031) and a grant from the Dutch Brain Foundation (project 2008(1).10). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Stroke 2003;34:2310–22. [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW1, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- 3. Wijnhoud AD, Maasland L, Lingsma HF, Steyerberg EW, Koudstaal PJ, Dippel DW Prediction of major vascular events in patients with transient ischemic attack or ischemic stroke: a comparison of 7 models. Stroke 2010;41:2178–85. 10.1161/STROKEAHA.110.580985 [DOI] [PubMed] [Google Scholar]
- 4. Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, et al. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol 2008;64:402–9. 10.1002/ana.21480 [DOI] [PubMed] [Google Scholar]
- 5. Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet 2009;41:876–8. 10.1038/ng.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gschwendtner A, Bevan S, Cole JW, Plourde A, Matarin M, Ross-Adams H, et al. Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann Neurol 2009;65:531–9. 10.1002/ana.21590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellenguez C, Bevan S, Gschwendtner A, Spencer CC, Burgess AI, Pirinen M, et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet 2012;44:328–33. 10.1038/ng.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE Collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol 2012;11:951–62. 10.1016/S1474-4422(12)70234-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pruissen DM, Kappelle LJ, Rosendaal FR, et al. Genetic association studies in ischaemic stroke: replication failure and prospects. Cerebrovasc Dis 2009;27:290–4. 10.1159/000199467 [DOI] [PubMed] [Google Scholar]
- 10. Achterberg S, Kappelle LJ, Algra A. Prognostic modelling in ischaemic stroke study, additional value of genetic characteristics. Rationale and design. Eur Neurol 2008;59:243–52. 10.1159/000115638 [DOI] [PubMed] [Google Scholar]
- 11. Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y. Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol 1999;15(9):773–81. [DOI] [PubMed] [Google Scholar]
- 12. Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res Ther 2011;13:101 10.1186/ar3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, et al. Integrating common and rare genetic variation in diverse human populations. Nature 2010;467:52–8. 10.1038/nature09298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. 10.1038/nature08185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Wijk I, Kappelle LJ, van Gijn J, Koudstaal PJ, Franke CL, Vermeulen M, Gorter JW, et al. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet 2005;365:2098–104. [DOI] [PubMed] [Google Scholar]
- 17. Sharma P, Yadav S, Meschia JF. Genetics of ischaemic stroke. J Neurol Neurosurg Psychiatry. 2013;84:1302–8. 10.1136/jnnp-2012-304834 [DOI] [PubMed] [Google Scholar]
- 18. Kilarski LL, Achterberg S, Devan WJ, Traylor M, Malik R, Lindgren A,et al. Meta-analysis in more than 17,900 cases of ischemic stroke reveals a novel association at 12q24.12. Neurology. 2014;83:678–85 10.1212/WNL.0000000000000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuzniatsova N1, Shantsila E, Blann A, Lip GY (2012) A contemporary viewpoint on 'aspirin resistance'. Ann Med 2012;44:773–83. 10.3109/07853890.2011.605388 [DOI] [PubMed] [Google Scholar]
- 20. Eikelboom JW, Emery J, Hankey. The use of platelet function assays may help to determine appropriate antiplatelet treatment options in a patient with recurrent stroke on baby aspirin: against. Stroke 2010;41:2398–9. 10.1161/STROKEAHA.110.593582 [DOI] [PubMed] [Google Scholar]
- 21. Alberts MJ. Platelet function testing for aspirin resistance is reasonable to do: yes! Stroke 2010;41:2400–1. 10.1161/STROKEAHA.110.595637 [DOI] [PubMed] [Google Scholar]
- 22. Gurbel PA, Tantry US. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents?: platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. Circulation 2012;125:1276–87. 10.1161/CIRCULATIONAHA.111.031195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.