Abstract
There is increasing evidence that the complement system plays an important role in diabetes and the development of diabetic vascular complications. In particular, mannan-binding lectin (MBL) levels are elevated in diabetes patients, and diabetes patients with diabetic nephropathy have higher MBL levels than diabetes patients with normal renal function. The MBL-associated serine proteases (MASPs) MASP-1, MASP-2 and MASP-3 and MBL-associated protein MAp44 have not yet been studied in diabetes patients. We therefore measured plasma levels of MASP-1, MASP-2, MASP-3 and MAp44 in 30 children with type 1 diabetes mellitus (T1DM) and 17 matched control subjects, and in 45 adults with T1DM and 31 matched control subjects. MASP-1 and MASP-2 levels were significantly higher in children and adults with T1DM than in their respective control groups, whereas MASP-3 and MAp44 levels did not differ between patients and controls. MASP-1 and MASP-2 levels correlated with HbA1c, and MASP levels decreased when glycaemic control improved. Because MASP-1 and MASP-2 have been shown to interact directly with blood coagulation, elevated levels of these proteins may play a role in the enhanced thrombotic environment and consequent vascular complications in diabetes.
Keywords: diabetes, mannan-binding lectin-associated serine proteases, MASP-1, MASP-2
Introduction
There is increasing evidence that the complement system, which is part of the innate immune system, plays an important role in diabetes and the development of diabetic vascular complications (please refer to the excellent reviews by Phieler et al. 1 and Hertle et al. 2). Plasma concentrations of several proteins of the complement system are elevated in patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), including the central component C3 3,4. Furthermore, deposits of complement activation products have been found in tissues from diabetes patients 5,6. Many studies have shown that high levels of circulating complement factors and increased complement activation are associated with vascular complications of diabetes such as cardiovascular disease and diabetic nephropathy.
We have an interest in the lectin pathway of the complement system, which may play a role in the development of these complications. Mannan-binding lectin (MBL) and ficolins are the pattern recognition molecules in the lectin pathway. Upon binding of MBL or ficolins to a target molecule, activation of the lectin pathway is mediated by the MBL-associated serine proteases (MASPs) MASP-1 and MASP-2. The role of MASP-3 is not yet fully understood 7–9. Two MBL-associated proteins, MAp19 and MAp44, which are alternative splicing variants of the MASP2 and MASP1 genes, respectively, have no protease activity. MAp44 was reported to have a regulatory function by displacing MASPs from the MBL complex and inhibiting lectin pathway activation 10,11.
Of the proteins mentioned above, MBL and ficolins have been studied so far in diabetes. MBL levels are elevated in patients with T1DM 12 and T2DM 13. Among diabetes patients, MBL and H-ficolin levels were higher in patients with diabetic nephropathy than in patients with normal renal function 14–16. In a prospective study, high MBL levels were associated with progression to end-stage renal disease 17.
This evidence for a role of MBL in diabetes and diabetic vascular complications inevitably leads to the question of whether or not levels of MASPs are also altered in diabetes and may contribute to diabetes complications. In the lectin pathway, MBL acts through its associated serine proteases, and MASPs circulate in plasma to a major extent in complex with MBL; yet MASP levels have never been measured in diabetes patients. To our knowledge, there is only one study available in which MASP-2 levels were measured in patients with T2DM who suffered from myocardial infarction 18. Patients were then followed for a median period of 2·1 years for further cardiovascular events. Patients who suffered from cardiovascular events during that period had significantly lower MASP-2 levels at admission, but the significant association was lost after adjustment for cardiovascular risk factors. We have shown earlier that levels of MASP-1 and MASP-2 are altered in patients with cardio- and cerebrovascular diseases 19.
The aim of our present study was to measure plasma levels of MASP-1, MASP-2, MASP-3, and MAp44 for the first time in patients with T1DM and investigate possible associations with glycaemic control.
Materials and methods
Patients and control subjects
We included 30 children and 45 adults with T1DM, and 17 children and 31 adults were included as non-diabetic, age- and sex-matched controls. All diabetes patients and control subjects were recruited at the University of Leeds. Control subjects were recruited through advertisement in the same hospital and unit where the patients were recruited. Other than background retinopathy, there were no significant microvascular complications in patients with T1DM and none were on any treatment other than insulin. We aimed to improve glycaemic control by adjusting insulin doses and regular patient contact, and collected repeat blood samples during the adult patients' routine clinic follow-up, usually occurring in 3–4 months. Due to loss to follow-up or missed appointments, repeat blood samples were obtained from only 26 adult patients.
Blood sampling was performed mid-morning after a light breakfast. Citrated plasma was separated within 2 h of collection and stored frozen in aliquots until analysis. All participants, and in the case of underage individuals also their parents, gave written informed consent in accordance with the Declaration of Helsinki. The study was approved by the local ethics committee.
Laboratory measurements
We measured levels of MASP-1, MASP-2, MASP-3, and MAp44 in citrated plasma samples which had been stored frozen in aliquots at −80°C until analysis. MASP-1 was determined with a competition enzyme-linked immunosorbent assay (ELISA) using a MASP-1-specific antibody, as described earlier 20. Plasma levels of MASP-2 and MASP-3 were measured with commercial ELISA kits (Hycult Biotech, Uden, the Netherlands). MAp44 was determined with a time-resolved immunofluorometric assay (TRIFMA) using a catching antibody and a biotinylated detecting antibody in a sandwich-type assay, as described previously 10. Interassay coefficients of variance of all assays were below 15%. Routine parameters were determined in the routine diagnostic laboratories of the Leeds General Infirmary hospital.
Statistical analysis
Statistical analysis was performed with IBM spss software, version 21. We used Kolmogorov–Smirnov and Shapiro–Wilk tests to check the data for normal distribution. Because most parameters did not follow the normal distribution in all groups, all data are displayed as median with interquartile range (25th percentile; 75th percentile). The appropriate parametric or non-parametric statistical tests were applied as indicated. Bivariate correlations of parameters were analysed using Pearson's or Spearman's correlation coefficients. Parameters were compared between two or multiple groups using the appropriate parametric [t-test or analysis of variance (anova)] or non-parametric (Mann–Whitney or Kruskal–Wallis tests) methods. Differences between paired samples were tested by Wilcoxon's or Friedman's tests. The χ2 test was used to compare categorical parameters between groups. A P-value of less than 0·05 was considered statistically significant.
Results
Characterization of diabetes patients and control subjects
Our study population comprised the following groups: (1) children with T1DM and healthy control subjects matched for age and sex; and (2) adults with T1DM and healthy control age- and sex-matched subjects. Demographic and clinical characteristics of these groups are shown in Tables 1 and 2.
Table 1.
Characteristics and plasma levels of mannan-binding lectin-associated serine proteases (MASPs) in children with type 1 diabetes mellitus (T1DM) and matched controls
| T1DM children | Controls | P-value* | |
|---|---|---|---|
| n = 30 | n = 17 | ||
| Age (years) | 14·9 (12·7; 15·6) | 14·4 (11·9; 15·4) | 0·319 |
| Weight (kg) | 58·7 (52·4; 63·5) | 50·8 (39·9; 60·2) | 0·068 |
| BMI (kg/m2) | 21·9 (20·6; 22·9) | n.d. | – |
| Total cholesterol (mmol/l) | 4·6 (4·0; 5·2) | n.d. | – |
| Diabetes duration (months) | 73·1 (34·2; 104·2) | n.a. | – |
| HbA1c (%) | 9·3 (8·1; 10·1) | n.d. | – |
| MASP-1 (μg/ml) | 11·1 (9·3; 13·7) | 7·9 (6·2; 11·5) | 0·007 |
| MASP-2 (ng/ml) | 420·9 (325·5; 509·8) | 278·4 (201·3; 423·2) | 0·008 |
| MASP-3 (μg/ml) | 8·2 (7·0; 9·3) | 7·6 (7·1; 9·2) | 0·364 |
| MAp44 (μg/ml) | 1·9 (1·5; 2·1) | 1·8 (1·4; 1·9) | 0·298 |
Continuous data are shown as median (25th percentile; 75th percentile). Map44 = mannose-binding lectin-associated protein; n = number; n.d. = not determined; n.a. = not applicable; BMI = body mass index.
Mann–Whitney U-test; HbAlc = glycated haemoglobin type A1c.
Plasma levels of MASPs and MAp44 in diabetes patients and controls
MASP-1 and MASP-2 levels were significantly higher in children (Table 1) and adults (Table 2) with T1DM than in their respective control groups, whereas MASP-3 and MAp44 levels did not differ between patients and controls. When we compared MASP levels between all groups, MASP-1 levels were lowest in non-diabetic children and highest in adults with T1DM (Fig. 1a). MASP-2 levels were lowest in non-diabetic children and young adults, and highest in adults with T1DM (Fig. 1b). MASP-3 levels did not follow this trend, and were lower in adults than in children irrespective of T1DM (Fig. 1c).
Table 2.
Characteristics and plasma levels of mannan-binding lectin-associated serine proteases (MASPs) in adults with type 1 diabetes mellitus (T1DM) and matched controls
| T1DM adults | Controls | P-value | |
|---|---|---|---|
| n = 45 | n = 31 | ||
| Age (years) | 22·0 (19·0; 26·0) | 23·0 (22·0; 26·0) | 0·390* |
| Weight (kg) | 71·9 (64·7; 78·8) | 71·0 (63·0; 80·0) | 0·747* |
| BMI (kg/m2) | 23·2 (20·8; 25·7) | 23·2 (21·7; 25·0) | 0·865* |
| Smoking, yes : no (%) | 8:36 (18:82) | 1:30 (3:97) | 0·102† |
| Microvascular complications, yes : no (%) | 10:31 (24:76) | 0:31 (0:100) | 0·004† |
| Creatinine (μmol/l) | 89·5 (82·0; 100·0) | 89·0 (79·0; 95·0) | 0·273* |
| Total cholesterol (mmol/l) | 4·4 (3·9; 5·2) | 4·1 (3·7; 5·0) | 0·142* |
| Diabetes duration (months) | 108·0 (55·5; 162·0)) | n.a. | – |
| HbA1c (%) | 9·0 (8·0; 9·9) | 5·4 (5·1; 5·5) | <0·001* |
| MASP-1 (μg/ml) | 12·5 (10·6; 15·5) | 9·7 (8·3; 13·6) | 0·003* |
| MASP-2 (ng/ml) | 400·1 (330·7; 580·8) | 290·4 (198·3; 390·1) | 0·001* |
| MASP-3 (μg/ml) | 4·8 (4·2; 5·6) | 5·6 (4·4; 6·7) | 0·058* |
| MAp44 (μg/ml) | 1·7 (1·5; 1·9) | 1·6 (1·5; 1·8) | 0·725* |
Continuous data are shown as median (25th percentile; 75th percentile). BMI = body mass index; Map44 = mannose-binding lectin-associated protein; n.a. = not applicable.
Mann–Whitney U-test
χ2 test; HbAlc = glycated haemoglobin type A1c.
Fig 1.
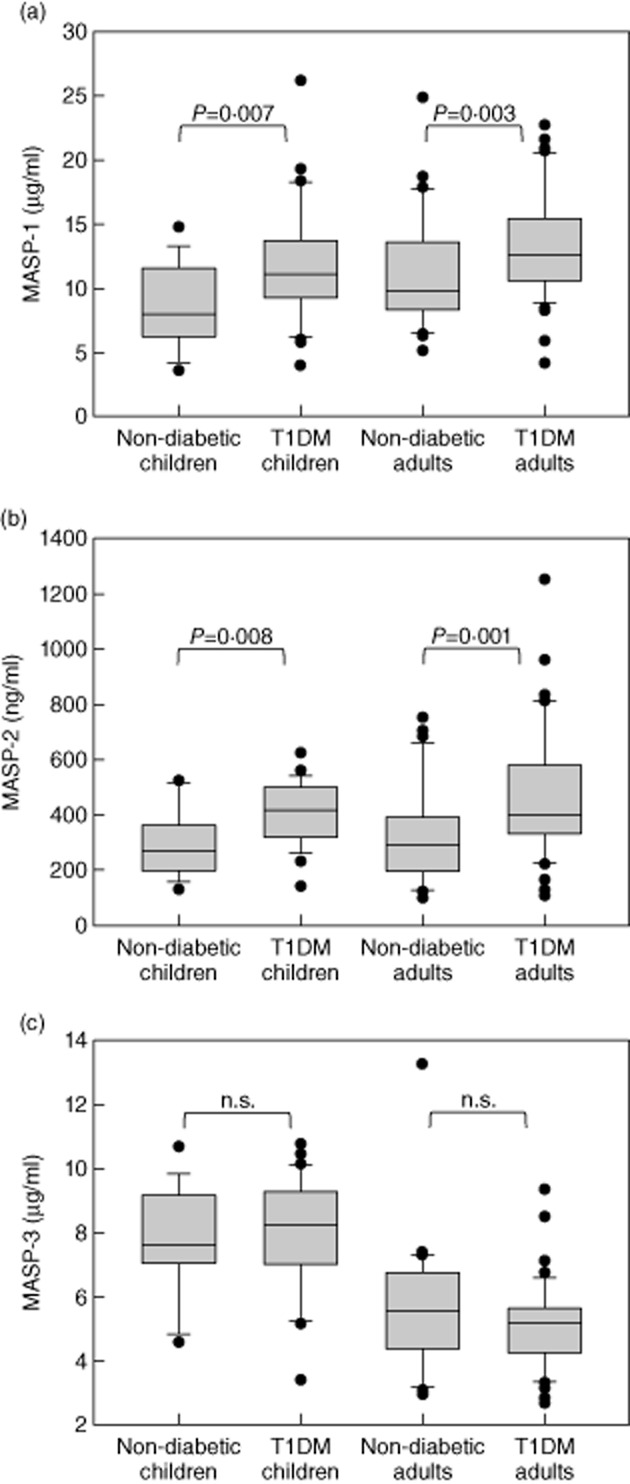
Plasma levels of mannan-binding lectin-associated serine proteases (MASPs) in patients with diabetes and control subjects showing (a) MASP-1, (b) MASP-2 and (c) MASP-3 levels. Boxes represent median, 25th and 75th percentiles, whiskers show the 10th and 90th percentiles and dots are values outside these percentiles. Groups were compared using the Mann–Whitney U-test and the P-value is indicated; n.s. = non-significant.
Effect of glycaemic control on levels of MASPs and MAp44
There were significant correlations between MASP-1 levels and HbA1c in children with T1DM [Spearman's correlation coefficient 0·456 (P = 0·011)] and in adults with T1DM (0·482, P = < 0·001). In adults with T1DM, there was also a correlation between MASP-2 levels and HbA1c (0·437, P = < 0·001), and both MASP-1 and MASP-2 levels correlated with the duration of T1DM (MASP-1 0·346, P = 0·004; MASP-2 0·359, P = 0·003). Neither MASP-3 nor MAp44 levels correlated with HbA1c in any patient group.
In a subgroup (n = 26) of adult patients with T1DM, we measured levels of MASPs and MAp44 at baseline and 16 ± 3 weeks after improving glycaemic control (shown in Table 3). Overall, a moderate but statistically significant reduction in HbA1c was not associated with significant reductions in MASP levels. However, patients whose HbA1c improved by at least 10% of the baseline value showed significant intra-individual reductions in MASP-1 and MASP-3. There was also a trend towards a reduction in MASP-2 levels, but the large variation in MASP-2 levels may be the reason for a non-significant result.
Table 3.
Effect of glycaemic control on plasma levels of mannan-binding lectin-associated serine proteases (MASPs) in adults with type 1 diabetes mellitus (T1DM)
| Baseline | After improvement of glycaemic control | P-value* for intra-individual change | |
|---|---|---|---|
| All patients in this subgroup (n = 26) | |||
| HbA1c (%) | 9·8 (9·2; 11·1) | 9·0 (7·9; 10·5) | 0·001 |
| MASP-1 (μg/ml) | 16·5 (14·8; 20·2) | 15·4 (13·4; 18·7) | 0·078 |
| MASP-2 (ng/ml) | 452·9 (315·7; 575·5) | 362·6 (237·3; 537·0) | 0·170 |
| MASP-3 (μg/ml) | 4·7 (4·1; 5·7) | 4·6 (3·9; 5·5) | 0·069 |
| MAp44 (μg/ml) | 2·0 (1·7; 2·2) | 1·8 (1·6; 2·1) | 0·054 |
| Patients whose glycaemic control improved most (HbA1c down by at least 10%) (n = 12) | |||
| HbA1c (%) | 10·4 (9·3; 14·1) | 8·2 (7·1; 9·7) | 0·002 |
| MASP-1 (μg/ml) | 16·9 (15·2; 21·5) | 14·9 (12·8; 18·5) | 0·012 |
| MASP-2 (ng/ml) | 487·1 (350·0; 649·7) | 441·5 (316·7; 635·9) | 0·136 |
| MASP-3 (μg/ml) | 5·3 (4·1; 6·5) | 4·8 (3·8; 5·4) | 0·011 |
| MAp44 (μg/ml) | 1·9 (1·7; 2·2) | 1·8 (1·7; 2·3) | 0·625 |
Data are shown as median (25th percentile; 75th percentile). n = number.
Wilcoxon's signed-rank test. Map44 = mannose-binding lectin-associated protein; HbAlc = glycated haemoglobin type A1c.
Taken together, these results suggest that blood glucose levels may represent a determinant of MASP levels, and in particular MASP-1 levels, in T1DM.
Discussion
Although MBL is well known to be elevated in diabetes patients and thought to be involved in vascular complications of diabetes, plasma levels of the MBL-associated serine proteases, MASP-1, MASP-2 and MASP-3, and the regulator MAp44, have not yet been studied in patients with diabetes. Here we show for the first time that MASP-1 and MASP-2 are elevated in patients with T1DM, that MASP-1 and MASP-2 levels correlate with HbA1c, and that glycaemic control may modulate MASP levels.
Our results suggest that levels of MASPs, in particular MASP-1 and MASP-2, may be linked to blood glucose levels. Recent data on MBL obtained in mouse models support this conclusion. MBL plasma levels were measured in mice before and 7 weeks after inducing diabetes by streptozotocin. Diabetes induction led to an increase in MBL-C that was associated with the increasing plasma glucose levels. This study suggested that MBL levels increase in mice as a consequence of diabetes 21. Other studies have shown that MBL knock-out or insulin treatment protected hyperglycaemic mice from cardiac complications 22,23.
A possible link between hyperglycaemia and complement activation has been suggested by Fortpied et al. 24. They could show that MBL binds with high affinity to the glycation product fructoselysine, and that this binding is associated with complement activation. Advanced glycation end-products (AGEs) have been considered responsible for various adverse outcomes associated with insulin resistance and diabetes, such as inflammatory processes, endothelial damage, activation of blood coagulation and vascular complications 25–27.
Based on the results of our present study we conclude that diabetes features not only elevate plasma levels of MBL, but also its associated serine proteases. The increase of MASPs in diabetes may be induced by the same underlying mechanisms that are responsible for the increase of MBL as a consequence of hyperglycaemia, or MASP levels increase secondary to the increase of MBL as their binding protein. The circulating complexes of MBL and MASPs bind to AGEs, and this induces conformational changes leading to activation of MASP-1 and consequently MASP-2. We and others have shown that both MASP-1 and MASP-2 interact directly with blood coagulation factors prothrombin, fibrinogen, factor XIII and thrombin-activatable fibrinolysis inhibitor, and hence can promote fibrin formation (as summarized recently by us 9). This could result eventually in thrombotic complications. We therefore hypothesize that the axis ‘hyperglycaemia → elevated levels of MBL, MASP-1, MASP-2 → binding to AGEs → activation of MASP-1 and MASP-2 → increased fibrin formation’ may represent an important link between diabetes and its thrombotic vascular complications.
Limitations of our study include the case–control design and the relatively small sample size. As there were no data in the literature on levels of MASPs and MAp44 in patients with diabetes, our intention was to perform this as a pilot study. The data presented in this work pave the way for future larger, prospective as well as mechanistic studies to determine the role of MASP levels and lectin pathway activation in the development of diabetes and its vascular complications, with the potential to discover new therapeutic targets.
Acknowledgments
This study was funded by the Swiss National Science Foundation and OPO Foundation (Zurich, Switzerland) (grants awarded to V. S.).
Disclosure
The authors have no financial or commercial conflicts of interest to declare.
Author contributions
L. J. and S. T. performed the laboratory measurements and analysed the data. R. A. and R. K. recruited and characterized the patients and controls and analysed the data. V. S. designed the study, analysed the data and wrote the manuscript. All authors revised the manuscript.
References
- Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013;25:47–53. doi: 10.1016/j.smim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle E, Stehouwer CDA, van Greevenbroek MMJ. The complement system in human cardiometabolic disease. Mol Immunol. 2014;61:135–148. doi: 10.1016/j.molimm.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Schroeder V, Carter AM, Dunne J, Mansfield MW, Grant PJ. Proinflammatory and hypofibrinolytic phenotype in healthy first-degree relatives of patients with type 2 diabetes. J Thromb Haemost. 2010;8:2080–2082. doi: 10.1111/j.1538-7836.2010.03966.x. [DOI] [PubMed] [Google Scholar]
- Hess K, Alzahrani S, Mathai M, et al. A novel mechanism for hypofibrinolysis in diabetes: the role of complement C3. Diabetologia. 2012;55:1103–1113. doi: 10.1007/s00125-011-2301-7. [DOI] [PubMed] [Google Scholar]
- Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2002;43:1104–1108. [PubMed] [Google Scholar]
- Uesugi N, Sakata N, Nangaku M, et al. Possible mechanism for medial smooth muscle cell injury in diabetic nephropathy: glycoxidation-mediated local complement activation. Am J Kidney Dis. 2004;44:224–238. doi: 10.1053/j.ajkd.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Yongqing T, Drentin N, Duncan RC, Wijeyewickrema LC, Pike RN. Mannose-binding lectin serine proteases and associated proteins of the lectin pathway of complement: two genes, five proteins and many functions? Biochim Biophys Acta. 2012;1824:253–262. doi: 10.1016/j.bbapap.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Héja D, Kocsis A, Dobó J, et al. Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci USA. 2012;109:10498–10503. doi: 10.1073/pnas.1202588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobó J, Schroeder V, Jenny L, Cervenak L, Závodszky P, Gál P. Multiple roles of complement MASP-1 at the interface of innate immune response and coagulation. Mol Immunol. 2014;61:69–78. doi: 10.1016/j.molimm.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Degn SE, Jensen L, Gál P, et al. Biological variations of MASP-3 and MAp44, two splice products of the MASP1 gene involved in regulation of the complement system. J Immunol Methods. 2010;361:37–50. doi: 10.1016/j.jim.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Banda NK, Mehta G, Kjaer TR, et al. Essential role for the lectin pathway in collagen antibody-induced arthritis revealed through use of adenovirus programming complement inhibitor Map44 expression. J Immunol. 2014;193:2455–2468. doi: 10.4049/jimmunol.1400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TK, Thiel S, Knudsen ST, et al. Elevated levels of mannan-binding lectin in patients with type 1 diabetes. J Clin Endocrinol Metab. 2003;88:4857–4861. doi: 10.1210/jc.2003-030742. [DOI] [PubMed] [Google Scholar]
- Mellbin LG, Hamsten A, Malmberg K, et al. Mannose-binding lectin genotype and phenotype in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care. 2010;33:2451–2456. doi: 10.2337/dc10-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TK, Tarnow L, Thiel S, et al. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes. 2004;53:1570–1576. doi: 10.2337/diabetes.53.6.1570. [DOI] [PubMed] [Google Scholar]
- Saraheimo M, Forsblom C, Hansen TK, et al. Increased levels of mannan-binding lectin in type 1 diabetic patients with incipient and overt nephropathy. Diabetologia. 2005;48:198–202. doi: 10.1007/s00125-004-1594-1. [DOI] [PubMed] [Google Scholar]
- Østergaard JA, Thiel S, Hovind P, et al. Association of the pattern recognition molecule H-ficolin with incident microalbuminuria in an inception cohort of newly diagnosed type 1 diabetic patients: an 18 year follow-up study. Diabetologia. 2014;57:2201–2207. doi: 10.1007/s00125-014-3332-7. [DOI] [PubMed] [Google Scholar]
- Hansen TK, Forsblom C, Saraheimo M, et al. Association between mannose-binding lectin, high-sensitivity C-reactive protein and the progression of diabetic nephropathy in type 1 diabetes. Diabetologia. 2010;53:1517–1524. doi: 10.1007/s00125-010-1742-8. [DOI] [PubMed] [Google Scholar]
- Mellbin LG, Bjerre M, Thiel S, Hansen TK. Complement activation and prognosis in patients with type 2 diabetes and myocardial infarction. Diabetes Care. 2012;35:911–917. doi: 10.2337/dc11-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenknecht V, Thiel S, Storm L, et al. Plasma levels of mannan-binding lectin (MBL)-associated serine proteases (MASPs) and MBL-associated protein in cardio- and cerebrovascular diseases. Clin Exp Immunol. 2013;173:112–120. doi: 10.1111/cei.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel S, Jensen L, Degn SE, et al. Mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1), a serine protease associated with humoral pattern-recognition molecules: normal and acute-phase levels in serum and stoichiometry of lectin pathway components. Clin Exp Immunol. 2012;169:38–48. doi: 10.1111/j.1365-2249.2012.04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard JA, Bjerre M, Dagnæs-Hansen F, Hansen TK, Thiel S, Flyvbjerg A. Diabetes-induced changes in mannan-binding lectin levels and complement activation in a mouse model of type 1 diabetes. Scand J Immunol. 2013;77:187–194. doi: 10.1111/sji.12027. [DOI] [PubMed] [Google Scholar]
- Busche MN, Walsh MC, McMullen ME, Guikema BJ, Stahl GL. Mannose-binding lectin plays a critical role in myocardial ischaemia and reperfusion injury in a mouse model of diabetes. Diabetologia. 2008;51:1544–1551. doi: 10.1007/s00125-008-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VI, La Bonte LR, Baldwin WM, Markiewski MM, Lambris JD, Stahl GL. Absence of mannose-binding lectin prevents hyperglycemic cardiovascular complications. Am J Pathol. 2012;180:104–112. doi: 10.1016/j.ajpath.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortpied J, Vertommen D, Van Schaftingen E. Binding of mannose-binding lectin to fructosamines: a potential link between hyperglycaemia and complement activation in diabetes. Diabetes Metab Res Rev. 2010;26:254–260. doi: 10.1002/dmrr.1079. [DOI] [PubMed] [Google Scholar]
- Kohler HP. Insulin resistance syndrome: interaction with coagulation and fibrinolysis. Swiss Med Wkly. 2002;132:241–252. doi: 10.4414/smw.2002.09856. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JBL. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. 2010;8:1663–1669. doi: 10.1111/j.1538-7836.2010.03910.x. [DOI] [PubMed] [Google Scholar]


