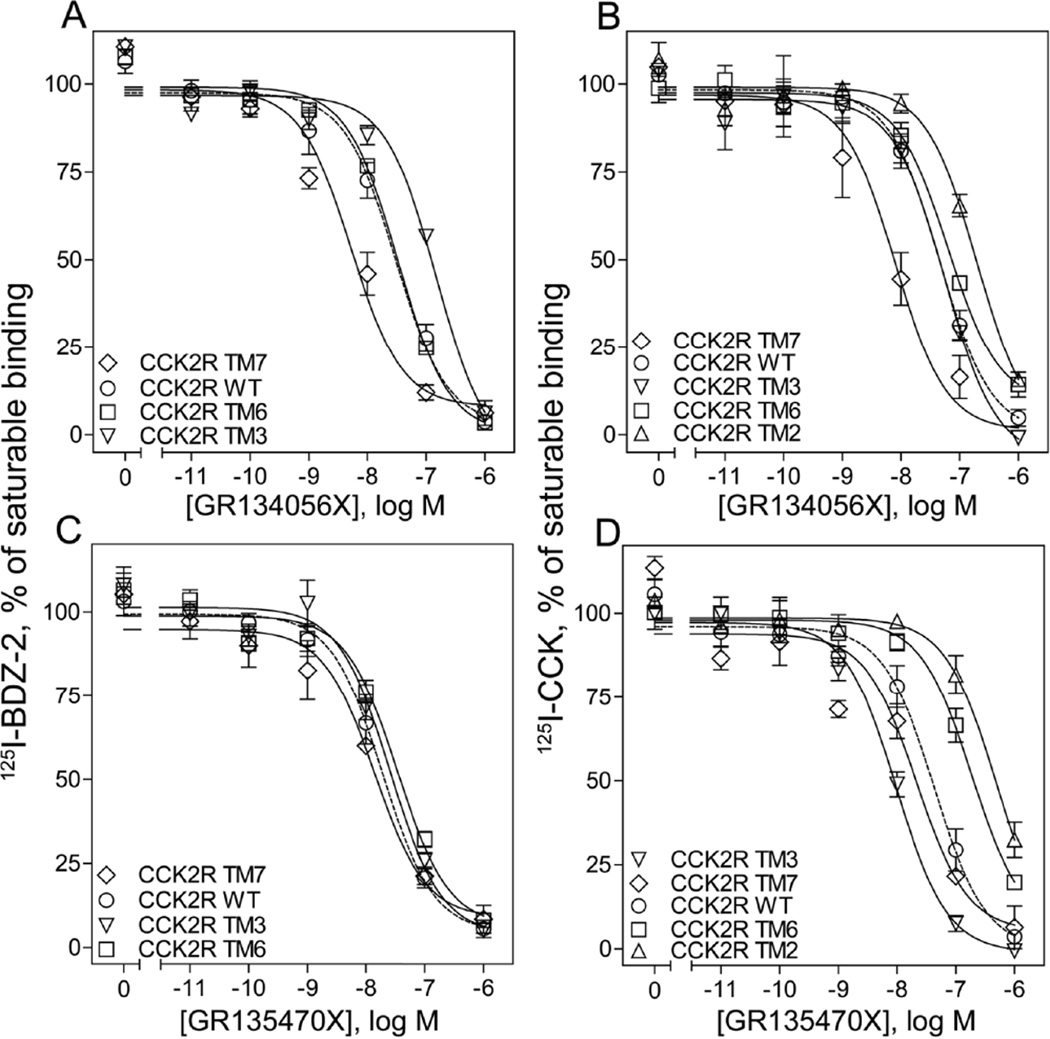
FIGURE 7.
Competition-binding studies using CCK2R-based chimeric constructs. Shown are the competition-binding curves for GR134056X and GR135470X using the allosteric antagonist radiolabel, 125I-BDZ-2 (A, C), or the orthosteric CCK-like radiolabel (B, D) to CHO cell membranes expressing CCK2R TM chimeric constructs, where specific residues for individual TM segments were exchanged with the corresponding residue of CCK1R. Values represent percentages of saturable binding that were observed in the absence of competitor. Non-saturable binding was determined by using 1µM unlabeled BDZ-2 or CCK. Data are expressed as means ± S.E.M. of duplicate determinations from at least three independent experiments.