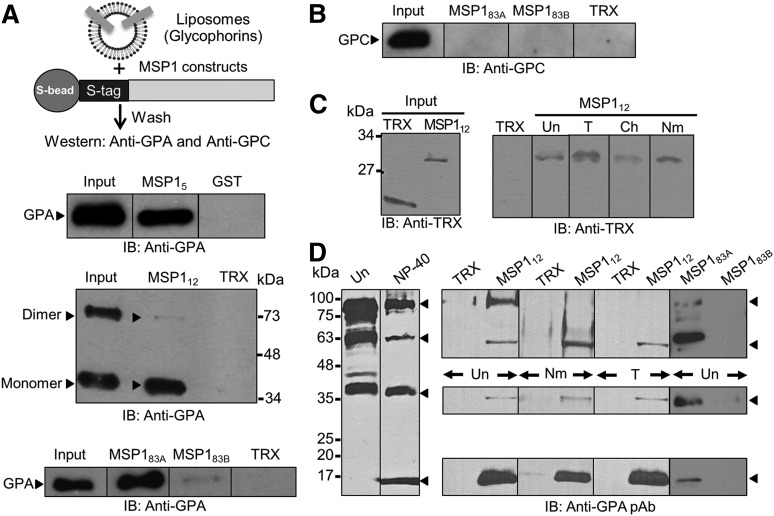
Figure 2.
MSP1-GPA binding assays. (A) MSP1 fusion proteins containing an S-tag (GST in MSP15) were immobilized to S-beads (or glutathione beads) and incubated with glycophorin-containing liposomes. Bound proteins were detected by immunoblotting (IB). The TRX fusion protein containing an S-tag and GST were used as negative controls. MSP112 showed specific binding to GPA relative to control in both the monomer (lower band) and dimer species of GPA (upper band). Binding of GPA dimer was detected under mild conditions of beads washings, whereas the GPA monomer binding was observed under both mild and stringent conditions. GPA binding to MSP183A and a very faint signal to MSP183B were also observed. (B) Immunoblotting against GPC did not detect any signal to either MSP183A or MSP183B fusion proteins. (C) RBC binding assay. Bound proteins were eluted by high salt and detected by immunoblotting. MSP112 showed specific binding to RBCs irrespective of their treatment with trypsin (T), chymotrypsin (Ch), or neuraminidase (Nm). Untreated RBCs (Un) were used as control. (D) Detection of GPA species in the detergent-solubilized human RBC ghosts by immunoblotting. Four specific bands of GPA were detected corresponding to homodimer, heterodimer, monomer, and a previously unrecognized 18-kDa band in the NP-40–solubilized ghosts. Immunoblotting using a polyclonal antibody (pAb) against GPA showed specific binding of MSP112 and MSP183A, but not MSP183B, to multiple species of GPA.