Abstract
AIM: To assess the effects of ME-49 Toxoplasma gondii (T. gondii) strain infection on the myenteric plexus and external muscle of the jejunum in rats.
METHODS: Thirty rats were distributed into two groups: the control group (CG) (n = 15) received 1 mL of saline solution orally, and the infected group (IG) (n = 15) inoculated with 1 mL of saline solution containing 500 oocysts of M-49 T. gondii strain orally. After 36 d of infection, the rats were euthanized. Infection with T. gondii was confirmed by blood samples collected from all rats at the beginning and end of the experiment. The jejunum of five animals was removed and submitted to routine histological processing (paraffin) for analysis of external muscle thickness. The remaining jejunum from the others animals was used to analyze the general population and the NADH-diaphorase, VIPergic and nitrergic subpopulations of myenteric neurons; and the enteric glial cells (S100-IR).
RESULTS: Serological analysis showed that animals from the IG were infected with the parasite. Hypertrophy affecting jejunal muscle thickness was observed in the IG rats (77.02 ± 42.71) in relation to the CG (51.40 ± 12.34), P < 0.05. In addition, 31.2% of the total number of myenteric neurons died (CG: 39839.3 ± 5362.3; IG: 26766.6 ± 2177.6; P < 0.05); hyperplasia of nitrergic myenteric neurons was observed (CG: 7959.0 ± 1290.4; IG: 10893.0 ± 1156.3; P < 0.05); general hypertrophy of the cell body in the remaining myenteric neurons was noted [CG: 232.5 (187.2-286.0); IG: 248.2 (204.4-293.0); P < 0.05]; hypertrophy of the smallest varicosities containing VIP neurotransmitter was seen (CG: 0.46 ± 0.10; IG: 0.80 ± 0.16; P < 0.05) and a reduction of 25.3% in enteric glia cells (CG: 12.64 ± 1.27; IG: 10.09 ± 2.10; P < 0.05) was observed in the infected rats.
CONCLUSION: It was concluded that infection with oocysts of ME-49 T. gondii strain caused quantitative and plastic alterations in the myenteric plexus of the jejunum in rats.
Keywords: Enteric nervous system, Infectious diseases, Glial cells, Nitric oxide, Neuronal plasticity, Small intestine, Toxoplasmosis, Vasoactive intestinal peptide
Core tip: The authors assessed the effects of ME-49 Toxoplasma gondii (T. gondii) strain infection on the myenteric plexus and external muscle of the jejunum in rats. They found an uncommon result when T. gondii infection was evaluated in rats: death of myenteric neurons and enteric glial cells. In addition, the remaining neurons showed hypertrophy and the number of nitrergic neurons increased. These alterations were possibly responsible for hypertrophy of the external muscle observed in the jejunal wall. The strain (ME-49) and the life form (oocysts) of T. gondii used here were the determinants of all these findings.
INTRODUCTION
Several pathogens invade animals via the digestive tract. Some of these pathogens (viruses, bacteria, protozoans and helminths) are able to survive in the hostile environment of the intestinal lumen. However, others, such as the protozoan Toxoplasma gondii (T. gondii), break the barrier of the intestinal epithelium, invade the lamina propria (causing an inflammatory reaction) and migrate to the bloodstream to spread in the host organism, searching for sites to evade the immune system[1-4].
The consequences of oral infection with T. gondii may vary, depending on parasite genotype and host species, from asymptomatic infection to the development of several alterations that may lead to death of the host[1-3].
In rats, it is known that the intestinal mucosa still shows signs of injury, detected by histopathological analysis, even after T. gondii had crossed the intestinal barrier, spreading through the host organism, forming tissue cysts (chronic phase)[5]. In addition, components of the nervous system intrinsic to the digestive tract, the enteric nervous system (ENS), reveal signs of plasticity due to alterations induced by toxoplasmic infections in the intestinal wall. Therefore, available experimental studies carried out in rats[5-15] have shown that these plastic alterations depend on several factors such as strain; infectious stage (tachyzoites, bradyzoites, sporozoites) and inoculation route (oral or intraperitoneal) of the parasite; infection phase (acute or chronic) assessed; digestive tract region and group of nervous cells assessed. For instance, while chronic infection caused by tachyzoites from a genotype I strain (for the SAG2 gene) causes atrophy of cell bodies in ileal myenteric neurons[7], this same infection causes hypertrophy of cell bodies in colonic myenteric neurons[8].
It is also possible that other alterations can be mediated by enteric glial cells. These cells form a vast network throughout the gastrointestinal wall, especially where there are myenteric and submucosal plexi[16]. Enteric glial cells are small and star-like[17] and can be identified by the presence of specific proteins such as the glial fibrillary acidic protein, vimentin, glutamine synthetase and S100. They contain neurotransmitter precursors such as GABA and NO and express receptors for determined cytokines such as interleukin (IL)-1β, IL-6, TNFα, and neuropeptides such as neurokinin A and substance P after activation[17-19]. Due to these characteristics, they act together in the neuro-immune axis established in the intestinal wall, and are therefore able to modulate some motility functions and gastrointestinal secretions. However, just one study has assessed enteric glial cells during T. gondii infection[5].
Considering the lack of studies on the impact of the infection caused by genotype II strains on the jejunal myenteric plexus, this study was carried out to assess the possible alterations caused by oral infection with T. gondii oocysts (ME-49 strain, genotype II) in the jejunum of rats. Specifically, we evaluated the thickness of intestinal wall; quantitative and morphometric of the total population of myenteric neurons as well as three subpopulations: NADH-diaphorase positive - composed of mitochondria-rich neurons; Nitrergic - produce nitric oxide; VIPergic - produce vasoactive intestinal peptide; and the total population of enteric glial cells that express the cytoplasm structural cytoplasmic protein: S-100.
MATERIALS AND METHODS
The experimental protocol of this study was previously approved by the Ethics Committee in Research Involving Animal Experimentation from Paranaense University, Brazil (Protocol 12361/2008).
Animal care and use statement
The animal protocol was designed to minimize pain or discomfort to the animals. During the experiments, the rats was maintained in an air-conditioned room (approximately 25 °C), 12 h/12 h light/dark, with food and water ad libitum. Half of the animals (infected group) received, by intragastric gavage, 500 T. gondii sporulated oocysts in 1 mL of sterile saline solution. The rats in the control group received only sterile saline solution. Thirty-six days after infection, the rats were euthanized in a chamber saturated with halothane for tissue collection.
Experimental design
The study included thirty 60-day-old male Wistar rats (Rattus norvegicus), weight 258.5 ± 13.6 g, which were equally and randomly assigned into the control [control group (CG); n = 15] and infected [infected group (IG); n = 15] groups.
In order to obtain the oocysts, cats (Felis catus) were inoculated orally with tissue cysts of T. gondii (ME-49 strain, genotype II), isolated from infected mice (Mus musculus). Stools were collected for seven days. Oocysts were concentrated by the Sheather method and sporulated in sulfuric acid at 2%[20].
Each rat in the IG received, 500 T. gondii sporulated oocysts re-suspended in 1 mL of sterile saline solution orally, while rats in the CG received only saline solution. Both groups were maintained in an air-conditioned room (approximately 25 °C), and received commercial feed for rodents and water ad libitum.
Infection by T. gondii was confirmed by blood samples collected from all rats at the beginning and end of the experiment. The serum was submitted to the direct agglutination method[21] in order to verify the presence of serum anti-T. gondii antibodies. Sera were considered positive when titers were greater than 25.
Thirty-six days after infection, the rats were euthanized in a chamber saturated with halothane[22]. Necropsy was performed immediately and the jejunum was removed, using the following anatomic limits as reference: duodenojejunal flexure and ileocecal fold. Each intestinal segment was then measured, washed and underwent intestinal wall analysis techniques.
Histological analysis
A two-centimeter ring from the proximal jejunum of five animals was submitted to routine histological processing (paraffin). From each jejunum, four transversal semi-serial 4 μm-sections were stained with hematoxylin and eosin (HE). Images of the histological sections were captured by a high resolution digital camera coupled to a trinocular photomicroscope (× 20 objective). These images were analyzed by the Motic Image Plus version 2.0 in order to carry out 80 measurements of the external muscle thickness, distributed uniformly around the whole intestinal circumference.
Histochemical technique
Wholemount preparations containing the jejunal myenteric plexus of five animals from each group were submitted to the Giemsa technique to highlight the total neuronal population[23]. From these same animals, 5 cm jejunal segments were submitted to a modified NADH-diaphorase histochemistry technique. These segments were washed in Krebs solution and then immersed for 5 min in Krebs solution + 0.3% Triton X-100 and washed (2 × 10 min each) with Krebs solution and immersed for 45 min in an incubation medium containing in each 100 mL: 25 mL Nitro Blue Tetrazolium (Sigma, St. Louis, MO, United States); 25 mL phosphate buffer 0.1 mol/L, pH 7.3; 50 mL distilled water and 5 mg β-NADH (Sigma, St. Louis, MO, United States)[24]. The intestinal segments were then dissected using a stereo microscope with transillumination to remove the mucosa and submucosa in order to analyze the jejunal myenteric neuronal subpopulation rich in mitochondria (NADHd-p).
Immunohistochemical technique
Intestinal segments were washed with PBS 0.1 mol/L pH 7.4 and filled with Zamboni fixative solution[25] for 18 h at 4 °C. After fixation, segments were opened along the mesenteric edge and washed in 80% ethanol solution to remove the fixation agent, followed by dehydration in ethanol solutions with ascending concentrations (95%-100%), deorphanization in xylol and rehydration in ethanol solutions with descending concentrations (100%, 90%, 80%, 50%) and stored in PBS + sodium azide 0.04% at 4 °C. After microdissection, wholemount preparations with the mesenteric plexus of each animal from both groups were obtained, washed with PBS 0.1 mol/L + Triton 0.3% for 5 min, and incubated in protein blocking solution for 2 h. The wholemount preparations were then incubated separately in different solutions containing the rabbit primary antibodies: anti-VIP, anti-NOS1[26] and anti-S 100[27,28] in order to label VIPergic and nitrergic myenteric neurons and enteric glial cells, respectively. Wholemount preparations remained in a cold room (4 °C) for 48 h and then washed three times in PBS 0.1 mol/L for 5 min. Then they were incubated in solution containing donkey anti-rabbit secondary antibody conjugated with fluorescein (1:500) at room temperature and protected from light for 2 h. The preparations were then washed three times in PBS 0.1 mol/L for 5 min, mounted using PBS/glycerol (9:1) and stored in the fridge.
Quantitative analysis
We counted the total number of myenteric neurons from each rat in 120 400X-magnified fields under the microscope (for the GIEMSA and NADH-diaphorase techniques) or in 32 images captured by a high-definition digital camera coupled to a fluorescence microscope (for the immunohistochemistry NOS+). The result of this count was projected to one square centimeters and to the total area of the jejunum. Neurons positioned on the limits of each microscope field/image were counted in alternate fields/images. A similar procedure was adopted for counting the enteric glial cells. In this case, we counted all enteric glial cells (S-100+) present in twenty × 200 magnified images captured by a high-resolution camera coupled to a fluorescent microscope. The number found in the sample area was projected to 1 mm2.
Morphometric analysis
The area of the cellular body, cytoplasm and nucleus of 300 jejunal myenteric neurons from each animal was measured using the captured images.
Measurement of the VIP-IR varicosities of the myenteric plexus and of the cell body area of NOS-IR myenteric neurons was carried out using images captured by the AxioCam (Zeiss, Jena, Germany), a light microscope resolution camera coupled to a light microscope5 equipped with filters for immunofluorescence in a × 40 objective, then transferred to a microcomputer by the AxioVision 4.1 program. Image-Pro Plus 4.5.0.29 (Media Cybernetics, Silver Spring, MD, United States®). software was adopted during the morphometric analysis of varicosities. Areas with 400 varicosities were measured for each animal, totaling 2000 per group. Only the nerve fibers which were not inside the ganglia were analyzed[29]. Photomicrographs were obtained through images provided by a confocal microscope (LSM 5 Pascal, Zeiss®).
Statistical analysis
Data from neuronal counting were initially submitted to the Shapiro-Wilk test and those from the morphometric analyses were submitted to the D’Agostino Pearson test to verify distribution type. Data with normal distribution were expressed by mean ± SD, and those with free distribution were expressed by the median and percentiles (P25; P75). The Student t test was adopted to compare data between the control and experimental groups regarding independent samples (for data with normal distribution) and the Mann-Whitney for data with free distribution, and P values less than 0.05 were considered significant. Correlation analysis was verified with the Spearman nonparametric test. Analyses were carried out with statistics software[30]. The statistical methods in this study were reviewed by Professor Aristeu Vieira da Silva from State University of Feira de Santana (Universidade Estadual de Feira de Santana), Brazil, who is biomedical statistician and co-author of this paper.
RESULTS
The results of the serological test performed before infection showed that all rats from the CG and IG were IgM and IgG negative for T. gondii. At the end of the experiment, the serological test confirmed that animals from the IG were infected with T. gondii, while animals from the CG remained susceptible to infection. In addition, animals from the IG had loose stools when compared to animals from the CG.
At the end of the experiment, body mass of the animals in the IG was greater than that in the CG (P < 0.05). With regard to the size of the jejunum (length, width and area), infection did not cause any alterations (P > 0.05). However, the morphometric analysis of the intestinal wall revealed hypertrophy affecting jejunal muscle thickness (P < 0.05) in the IG compared with the CG (Table 1).
Table 1.
Initial and final weight; length, width and area of the jejunum and thickness of the external muscle layer of the jejunum in rats from the control group and infected group
| Parameters | CG | IG |
| Initial body weight (g) | 254.8 ± 15.90 | 262.4 ± 11.29 |
| Final body weight (g) | 394.1 ± 14.941 | 419.8 ± 31.531 |
| Length (cm) | 108.4 ± 5.07 | 110.4 ± 6.83 |
| Width (cm) | 0.96 ± 0.15 | 0.96 ± 0.15 |
| Area (cm2) | 104.35 ± 19.15 | 106.82 ± 23.53 |
| Muscular fold thickness (μm) | 51.40 ± 12.341 | 77.02 ± 42.711 |
Significantly different (P < 0.05). IG: Infected group; CG: Control group.
Infected animals showed death of neurons according to quantitative analyses of the myenteric population stained using the Giemsa technique. However, the number of NADHd-p neurons was unaltered. In addition, an increase in nitrergic myenteric neurons in the IG was observed (Table 2 and Figure 1).
Table 2.
Population density and morphometric analysis of the cell body of myenteric neurons labeled with Giemsa, NADHd-p and NOS immunohistochemistry in healthy and infected rats with the ME-49 strain of Toxoplasma gondii during 36 d
| Measures |
GIEMSA |
NADHd-p |
NOS |
|||
| CG | IG | CG | IG | CG | IG | |
| Number of neurons/cm2 | 39839.3 ± 5362.31 | 26766.6 ± 2177.61 | 11484.5 ± 2211.8 | 13155.9 ± 1319.3 | 7959.0 ± 1290.41 | 10893 ± 1156.31 |
| Projection of number of neurons for the organ | 4157229.4 ± 559555.11 | 2859215.3 ± 232611.31 | 1198410.1 ± 230807.1 | 1405318.8 ± 157570.2 | 735729.9 ± 125754.71 | 939630.1 ± 156871.21 |
| Cell body (μm2) | 232.5 (187.2-286.0)1 | 248.2 (204.4-293.0)1 | 146.7 (96.8-202.0)1 | 155.8 (108.0-14.8)1 | 328.1 ± 2.431 | 344.1 ± 2.21 |
| Nucleus (μm2) | 99.9 (82.5-115.3)1 | 102.6 (88.5-119.2)1 | 64.9 (43.7-88.5)1 | 76.9 (54.1-99.2)1 | 97.5 ± 0.701 | 100.9 ± 0.671 |
| Cytoplasm (μm2) | 130.8 (99.4-175.1)1 | 141.6 (112.1-182.4)1 | 79.2 (48.0-115.9)1 | 74.2 (48.2-117.4)1 | 230.6 ± 2.11 | 243.8 ± 1.91 |
| Ratio | 0.42 (0.37-0.48) | 0.41 (0.37-0.47) | 0.44 (0.37-0.51)1 | 0.48 (0.40-0.57)1 | 0.30 ± 0.002 | 0.31 ± 0.003 |
For the same neuronal marker, are significantly different (t, P < 0.05). IG: Infected group; CG: Control group.
Figure 1.
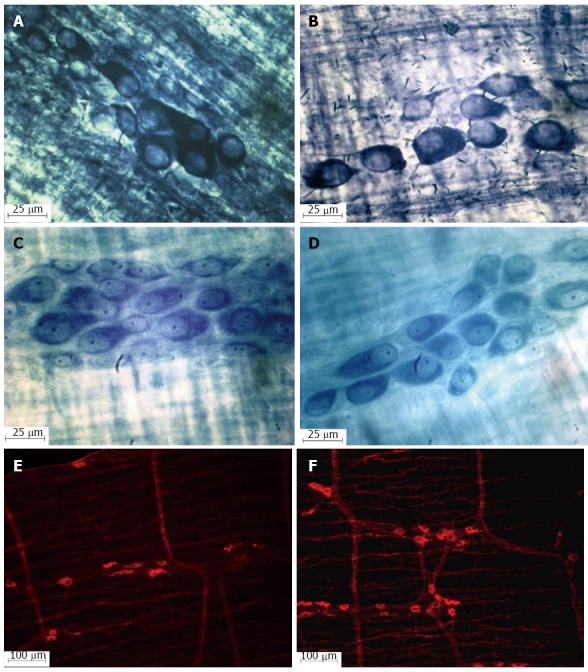
Photomicrograph of the myenteric ganglia in the jejunum of healthy (A, C and E) and infected rats (B, D and F); NADH-diaphorase (A and B); Giemsa (C and D). NOS-IR (E and F) showing the increase in the nitrergic myenteric neuron population in rats infected with oocysts of the ME-49 genotype II (F) strain of Toxoplasma gondii.
The morphometric analysis of the myenteric neurons showed that cellular bodies demonstrated slight hypertrophy in the total population as well as in the nitrergic and NADHd-p subpopulations (P < 0.05). For the total population, hypertrophy was due to an increase in the area of cytoplasm and nucleus; however, the proportion occupied by the nucleus inside the cell body was not compromised (P < 0.05). This same phenomenon was observed in nitrergic neurons (P < 0.05). Conversely, neurons from the subpopulation with more mitochondria inside the cell body (NADHd-p) showed an increase in the nucleus area and a reduction in the cytoplasm area, causing discrete atrophy of the cell body as well as an increase in the proportion occupied by the nucleus inside the cell body (P < 0.05) (Table 2).
The smallest areas of the VIPergic varicosities in the IG increased by 73.9% compared to the CG (P < 0.05); however, the larger and medium varicosities remained unaltered in animals from the IG (P < 0.05). In this study, enteric glial cell S-100 IR showed a quantitative alteration (P < 0.05) after infection. The total number of enteric glial cells was 1537 ± 38.7 in the CG and 1227 ± 60.8 in the IG in 1.52 mm² (P < 0.05). Thus, there was a 25.3% reduction in the animals from the IG (P < 0.05). The area of the VIP-IR varicosities found in the myenteric plexus and the enteric glial cell distribution are shown in Table 3.
Table 3.
Smaller, medium and larger areas (μm²) of VIPergic varicosities and number of enteric glial cells/mm² from the jejunal myenteric plexus of rats from the Control Group and from the Group infected with oocysts of Toxoplasma gondii ME-49 strain
| Parameters | CG | IG |
| Smaller areas (μm2) | 0.46 ± 0.101 | 0.80 ± 0.161 |
| Larger areas (μm2) | 21.68 ± 5.13 | 24.50 ± 0.90 |
| Medium areas (μm2) | 9.28 ± 5.84 | 9.51 ± 5.99 |
| Enteric glial cells/mm² | 1011.18 ± 25.481 | 807.24 ± 39.981 |
Significantly different (t, P < 0.05). IG: Infected group; CG: Control group.
DISCUSSION
Experimental infection induced by T. gondii (ME-49 strain, genotype II) oocysts was the cause of the alterations observed in the jejunal wall structure assessed in this study, mainly within the myenteric plexus. This finding is supported by the fact that animals from the CG remained healthy throughout the experiment, while animals from the IG showed anti-T. gondii serum IgG antibodies, indicating true infection by the parasite.
In general, animals are infected by ingesting tissue cysts of T. gondii present in raw or rare meat or oocysts found in contaminated food or water. When T. gondii crosses the gastrointestinal tract wall it can cause multifunctional alterations[31-33]. These alterations seem to be related to several factors such as genotype strain, life form and inoculation route (oral or intraperitoneal) of the parasite in addition to the infection phase (acute or chronic), digestive tract region and the type of cells assessed[5-15].
For the organ and host species assessed in this study, rat jejunum previously studied by our research group using oocysts of another strain (M7741 - genotype III), showed that T. gondii was capable of promoting plastic alterations in the enteric neurons without leading to neuronal death during the acute[10] or chronic[12] phases. This finding was different from that observed in the present study which demonstrated that strain ME-49 caused the death of 31.2% of total myenteric neurons. Myenteric neuronal death caused by toxoplasmic infection is not a common finding. Until now this phenomenon had only been observed in the duodenum of poultry[33] and the stomach of rats[11] infected by the T. gondii genotype III strain.
In addition, the proportion of nitrergic myenteric neurons increased from 17.7% in the CG to 32.8% in the IG. The number of nitrergic enteric neurons found in CG animals corresponds to 18% of the total myenteric neurons described in the literature[34]. An increase in the proportion of nitrergic myenteric neurons was also observed in the jejunum of pigs infected with the M7741 (genotype III) strain of T. gondii[35], but not in the jejunum of rats infected with the same strain[12]. This shows that the ME-49 strain of T. gondii used in the present study was a determinant of the death of myenteric neurons in rat jejunum.
The NADHd-p jejunal myenteric neurons analyzed in this study showed no alterations due to infection; however, there was a reduction of 50% in these cells in the jejunum of pigs infected with the M7741 (genotype III) strain of T. gondii[35]. Rats infected with the M7741 (genotype III) strain of T. gondii also showed no alteration in the population density of NADHd-p myenteric neurons[10]. These results reinforce the fact that observable alterations in the myenteric plexus of animals infected with T. gondii depend on genotype strain, life form and inoculation route (oral or intraperitoneal) of the parasite in addition to the infection phase (acute or chronic), digestive tract region and the type of neurons assessed. This explains the diarrhea seen in some species when infected with T. gondii, while in others this clinical sign is not observed. However, some infected animals which do not exhibit diarrhea may develop constipation. In addition, some of the cases considered “asymptomatic” could represent a misunderstanding caused by lack of attention given to possible intestinal constipation. Further studies are necessary to assess the intestinal motility of animals infected by T. gondii.
The modifications in the myenteric neuronal density observed in rats from the IG may be related to the hypertrophy observed in 49.80% of the external muscle layer (Figure 2), as these neurons innervate this muscle. The death of myenteric neurons may trigger several functional disorders directly or indirectly[36-39]. Our data suggest that hypertrophy of the external muscle layer was a compensation mechanism due to neuronal loss in order to maintain the jejunum of the infected animals in an adequate condition for the digestion and absorption of nutrients. It is interesting to observe that despite morphological alterations in the intestine after 36 d of infection, our results show that the rats continued developing including gaining body mass. In the jejunum of pigs, M7741 (genotype III) of the T. gondii caused atrophy of the external muscle layer after 30 d of infection and then hypertrophy 60 d after infection[40]. Hypertrophy of the external muscle layer was also found in the jejunum and ileum of chicken infected with ME-49 tissue cysts of T. gondii and in the jejunum of these same animals when infected with oocysts of the M7741 strain[32,41]. There are no previous studies in the literature assessing the effects of T. gondii infection on the external muscle layer of the intestine in rats.
Figure 2.
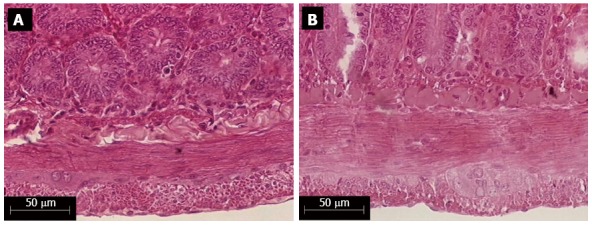
External muscle layer thickness of the jejunal wall of healthy (A) and infected rats (B) with the ME-49 genotype II strain of Toxoplasma gondii, colored by the HE histological technique.
The morphometric analysis of myenteric neurons revealed that, even after 36 d of infection, these cells showed hypertrophied cell bodies in both the total population as well as in the subpopulations assessed in this study. It should be emphasized that the hypertrophy observed in the cell body of the total population and nitrergic subpopulation was the result of an increase both in the nucleus area as well as in the area of the cytoplasm. On the other hand, an increase in the nuclear area (18.5%) and a reduction in the cytoplasmic area (5.3%) were observed in the NADHd-p population, leading to slight hypertrophy of their cell body.
Considering that the results from the present study showed different plasticity between NADHd-p and nitrergic myenteric neurons and that the myenteric plexus carries subpopulations of inhibitory (nitrergic/VIPergic) and excitatory (cholinergic) motor neurons[42], it is possible to infer that a considerable portion of NADHd-p neurons assessed in this study belongs to the subpopulation of cholinergic myenteric neurons. This same rationale can be applied to the population density assessment, as the increase in the proportion of nitrergic myenteric neurons (from 17.7% to 32.8% of the total) observed in animals infected by T. gondii suggests a reduction in the number of cholinergic neurons from 82.3% to 62.3% of the total. In this context, it is possible that the increase (18.5%) in the nuclear area of the NADHd-p myenteric neurons (possibly cholinergic, as discussed above) may be related to an increase in metabolic activity of cholinergic myenteric neurons seen as a compensatory effect in relation to the reduction in population density of these cells.
The increase in population density of nitrergic myenteric neurons along with their hypertrophy shows that infection caused by ME-49 strain T. gondii provoked a plastic alteration in a large part of the jejunal myenteric plexus of infected rats. Within this context we can also include the increase observed in the smaller varicosities with VIP of nerve fibers of the myenteric plexus of infected animals. As these fibers with VIP belong, mainly, to inhibitory motor neurons which also produce nitric oxide (NO)[43,44], it is suggested that the increase in the varicosities is related to an increase in NO production, thus potentializing the effects of the inhibitory motor route to recover the homeostasis of intestinal motility when looser stools were detected in infected animals. On the other hand, a parallel study with the same animals in this study showed a reduction of 28.4% in VIP-IR submucous neurons in the submucous plexus and atrophy of their cell bodies[5].
Another approach adopted by this study included the quantification of enteric glial cells which showed a reduction in these cells (P < 0.05) in the myenteric plexus of the jejunum of rats infected with T. gondii. It can be inferred that the loss of the neuronal population by 32% occurred due to a reduction in enteric glial cells. These cells play an important neurotrophic, anti-apoptotic[45-47] and neuromuscular transmitter role[42,43,36]. As a reduction of a little more than 25% of glial S-100 IR cells in the myenteric plexus of the jejunum in rats was demonstrated in this study, it is suggested that the alterations observed occurred due to the death of glial cells.
Therefore, we believe that the death of enteric glial cells was a determining factor in the changes in the metabolic profile and chemical code of the remaining neurons. This finding is supported by the fact that there were changes in the phenotypic profile in the subpopulations that predominate in the myenteric plexus and that these changes in expression would have caused an increase in the proportion of inhibitory motor neurons (nitrergic) (from 17.7% to 32.8% of the total). However, with the increase in the number of nitrergic neurons, there was an increment in NO production which may induce the release of NO by the neurons[48]. This study describes an increase of 73% in the smallest areas of the VIPergic varicosities in the myenteric plexus of animals from the IG and we question whether the increase in the smallest varicosities of the VIPergic fibers and consequently their greater expression in the myenteric plexus occurred through this mechanism (Figure 3).
Figure 3.
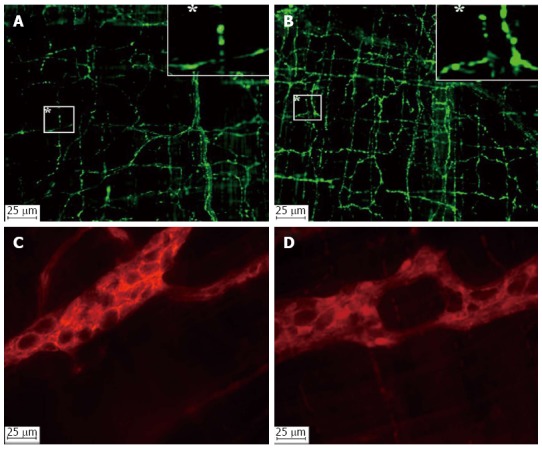
Photomicrographs of the VIPergic fibers (A and B) and enteric glial cells (C and D) in the jejunal myenteric plexus of healthy (A and C) and infected (B and D) rats with the ME-49 genotype II strain of Toxoplasma gondii.
VIP is an anti-inflammatory neuropeptide[49,50], therefore its increase may be related to modulation of the inflammatory reaction and recruitment of cells that will act upon tissue repair. It is believed that through this mechanism the enteric glial cells play a central role as mediators of multidirectional interactions among neurons and the immune system[18,51], which in a dynamic way contributes to the homeostasis of the whole gastrointestinal tract[52]. However, as there are no other studies assessing enteric glial cells during infection by T. gondii[5], we are unable to compare our results.
It is possible that all these modifications in the myenteric plexus were established after the death of 32% of the total neurons and 25% of the total enteric glial cells in this region of the digestive tract. It may be that there was effectivity in all the observed plastic alterations, as the infected animals continued developing with body mass gains, indicating that digestion and absorption of nutrients were not affected.
However, it is important to highlight that atrophy of the cell body in nitrergic myenteric neurons in the jejunum has been observed in rats infected with oocysts of the T. gondii: strain M7741[12]. A comparison between these findings and the results from our present study suggest that the ME-49 strain is more virulent to the myenteric plexus neurons of rat jejunum. For the population of nitrergic myenteric neurons, the T. gondii M7741 strain was capable of causing, in the jejunum of pigs[35], reactions similar to those observed in our study with the ME-49 strain, in rats. However, with regard to the NADHd population, pigs infected by strain M7741 showed the opposite result when compared to rats infected by the ME-49 strain.
This is the first study to assess enteric glial cell reaction to this protozoal infection. New studies are necessary to compare results and understand the questions raised here. Future studies on the assessment of the intestinal transit of animals infected by T. gondii strains need to be carried out to understand the impact of plastic alterations and population density in the myenteric plexus on the motility of different regions of the digestive tract.
COMMENTS
Background
Toxoplasmosis is one of the most prevalent infectious diseases worldwide. It is estimated that 40% to 70% of the world population is infected with the causative agent of the disease: Toxoplasma gondii (T. gondii). The oral route is the natural entry for this protozoan. In recent years, studies have shown that this parasite causes changes in the enteric nervous system (ENS) of different host species. The ENS is the intrinsic nervous system of the gastrointestinal tract. These changes are very heterogeneous and depend on the characteristics of the parasite and the host. The changes range from histopathological and plastic neural adaptations to the death of enteric neurons in the host. These changes are due to a number of influences linked to the parasite (genotype, inoculum size, inoculated life form, time of infection, etc.) and the host (species, region of the gastrointestinal tract and morphofunctional type of neuron). This study reports ENS changes caused by oocysts of the genotype II strain of T. gondii on the myenteric neurons of the jejunum in rats.
Research frontiers
The comprehension of intestinal tissue response to aggressive agents such as T. gondii is important in understanding functional and structural alterations in the gut. Inflammatory bowel diseases affect many people and models of infection with T. gondii are one of the ways to study these diseases. This research group is the first to investigate the relationship between the enteric nervous system and T. gondii. Previous studies by our group showed that genotype III strains promote morphological changes in enteric neurons of the jejunum, ileum and colon in rats and the jejunum in pigs. Previous studies performed using the ME-49 strain (genotype II) showed changes in the submucosal plexus of rats, especially in VIPergic neurons.
Innovations and breakthroughs
This study is the first to describe the effects of chronic infection with the genotype II strain of T gondii on different neuronal subpopulations of the myenteric plexus and related structures in the jejunum of rats. It contributes to the consolidation of an experimental model of this infectious disease and inflammatory bowel disease.
Applications
This study demonstrates that the ME-49 strain of T. gondii is extremely aggressive in the myenteric neurons of the jejunum in rats, as it was capable of causing death of some of these cells. Death of enteric neurons in rats infected with T. gondii is a uncommon finding.
Terminology
Toxoplasmosis is a disease characterized by clinical manifestations of infection caused by T. gondii. T. gondii is a protozoan that parasitizes warm-blooded animal cells. T. gondii has three life forms: tachyzoites (fast asexual reproduction, present in the acute phase of infection), bradyzoites (slow asexual reproduction, present in the chronic phase of infection) and sporozoites (present in oocysts which are the result of sexual reproduction of the parasite which only occurs in the intestine of felids). The oocysts are shed in the feces of felids and can contaminate food and water, which can be ingested by man and other animals leading to infection. The enteric nervous system is present in the wall of the digestive tract (esophagus to the anus). The cell bodies of these neurons are organized in two plexi. The submucosal plexus is localized in the submucosa and regulates the flow of blood and secretion of substances into the tube lumen. The myenteric plexus is present in the external muscle layer and regulates the food traffic in contact with the mucosa.
Peer-review
This paper assesses the inflammatory process in the digestive tract caused by T. gondii ME-49 strain infection. The objective of this study was to assess the possible effects of this parasite on the myenteric plexus and external muscle of the jejunum in rats. The findings suggest that infection by oocysts of ME-49 T. gondii strain caused quantitative and plastic alterations in the myenteric plexus of the jejunum in rats to maintain the homeostasis of the animals. This is a well-written paper containing interesting results.
Footnotes
Ethics approval: All procedures involving animals were reviewed and approved by the Ethics Committee in Research Involving Animal Experimentation of the Paranaense University (Universidade Paranaense), Brazil (Protocol 12361/2008).
Institutional animal care and use committee: All procedures involving animals were reviewed and approved by the Ethics Committee in Research Involving Animal Experimentation of the Paranaense University (Universidade Paranaense), Brazil (Protocol 12361/2008).
Conflict-of-interest: Eduardo José de Almeida Araújo is an employee of State University of Londrina, Brazil. Jacqueline Nelisis Zanoni and Débora de Mello Gonçales Sant’Ana are an employee of State University of Maringá, Brazil. Aristeu Vieira da Silva is an employee of State University of Feira de Santana, Brazil. Eduardo José de Almeida Araújo has received research funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil; Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná, Brazil. Jacqueline Nelisis Zanoni has received research funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil; Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná, Brazil. Aristeu Vieira da Silva has received research funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil; Fundação de Amparo à Pesquisa do Estado da Bahia. Débora de Mello Gonçales Sant’Ana has received research funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil; Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná, Brazil.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 8, 2014
First decision: December 11, 2014
Article in press: January 30, 2015
P- Reviewer: Caviglia RD, Luo HS S- Editor: Qi Y L- Editor: Webster JR E- Editor: Ma S
References
- 1.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss L, Kim , K . Toxoplasma gondii: the model apicomplexan. Perspectives and methods. Alterations in host-cell biology due to Toxoplasma gondii. 1th ed. London: Elsevier; 2007. pp. 10–752. [Google Scholar]
- 3.Dubey JP, Beattie CP. Toxoplasmosis of Animals and Man. Boca Ratón, Florida: CRC Press; 1988. pp. 5–220. [Google Scholar]
- 4.Gregg B, Taylor BC, John B, Tait-Wojno ED, Girgis NM, Miller N, Wagage S, Roos DS, Hunter CA. Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect Immun. 2013;81:1635–1643. doi: 10.1128/IAI.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sant’Ana DM, Góis MB, Zanoni JN, da Silva AV, da Silva CJ, Araújo EJ. Intraepithelial lymphocytes, goblet cells and VIP-IR submucosal neurons of jejunum rats infected with Toxoplasma gondii. Int J Exp Pathol. 2012;93:279–286. doi: 10.1111/j.1365-2613.2012.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugauara EY, Sant’Ana Dde M, Almeida EC, Reis AB, Silva AV, Araújo EJ. Alterations of the myenteric plexus of the ileum and the descending colon caused by Toxoplasma gondii (genotype III) Arq Neuropsiquiatr. 2008;66:516–523. doi: 10.1590/s0004-282x2008000400015. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa BJP, Araújo EJA, Da Silva AV, Sant’Ana DMG. Atrofia neuronal mientérica do íleo de ratos infectados cronicamente por uma cepa genótipo III de Toxoplasma gondii. Arq Cien Vet Zool Unipar. 2009;12:101–108. [Google Scholar]
- 8.Soares J, Moreira NM, da Silva AV, Sant’Ana Dde M, Araújo EJ. [Chronic infection due to Toxoplasma gondii inducing neuron hypertrophy of the myenteric plexus of Rattus norvegicus descending colon] Rev Bras Parasitol Vet. 2009;18:57–60. doi: 10.4322/rbpv.01802013. [DOI] [PubMed] [Google Scholar]
- 9.Sugauara EYY, Sant’Ana DMG, Almeida EC, Souza EA, Silva AV, Araújo EJA. Hypertrophy of the neurons in the ileum from rats infected with cysts of Toxoplasma gondii (Genotype II) Acta Scient Biolog Sci. 2009;31:195–201. [Google Scholar]
- 10.Pereira LS, Silva AV, Araújo EJA, Sant’Ana DMG. Hypertrophy of NADH-diaphorase positive myenteric neurons in rat jejunum after acute infection caused by Toxoplasma gondii. J Venom Anim Toxins incl Trop Dis. 2010;16:298–310. [Google Scholar]
- 11.Alves MS, Silva AV, Bianchi LRO, Araújo EJA, Sant’Ana DMG. Toxoplasma gondii induces death of gastric myenteric neurons in rats. Int J of Morphol. 2011;29:293–298. [Google Scholar]
- 12.Hermes-Uliana C, Pereira-Severi LS, Luerdes RB, Franco CL, da Silva AV, Araújo EJ, Sant’Ana Dde M. Chronic infection with Toxoplasma gondii causes myenteric neuroplasticity of the jejunum in rats. Auton Neurosci. 2011;160:3–8. doi: 10.1016/j.autneu.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Silva LS, Sartori AL, Zaniolo LM, da Silva AV, Sant’Ana Dde M, Araújo EJ. Toxoplasma gondii: myenteric neurons of intraperitoneally inoculated rats show quantitative and morphometric alterations. Exp Parasitol. 2011;129:5–10. doi: 10.1016/j.exppara.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Papazian-Cabanas RM, Araújo EJ, Silva AV, Sant’Ana DM. Myenteric neuronal plasticity induced by Toxoplasma gondii (genotype III) on the duodenum of rats. An Acad Bras Cienc. 2012;84:737–746. doi: 10.1590/s0001-37652012005000052. [DOI] [PubMed] [Google Scholar]
- 15.Zaniolo LM, da Silva AV, Sant’Ana Dde M, Araújo EJ. Toxoplasma gondii infection causes morphological changes in caecal myenteric neurons. Exp Parasitol. 2012;130:103–109. doi: 10.1016/j.exppara.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 17.De Winter BY, De Man JG. Interplay between inflammation, immune system and neuronal pathways: effect on gastrointestinal motility. World J Gastroenterol. 2010;16:5523–5535. doi: 10.3748/wjg.v16.i44.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rühl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17:777–790. doi: 10.1111/j.1365-2982.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 19.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 20.Sloss MW, Zajac AM, Kemp RL. Parasitologia Clínica Veterinária. São Paulo: Manole; 1999. pp. 5–198. [Google Scholar]
- 21.Desmonts G, Remington JS. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol. 1980;11:562–568. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivas LA, Jamel N, Refinetti RA, Silva LF, Rodrigues LV, Silva PC, Schanaider A. Anesthetic experimental device for small animal. Acta Cir Bras. 2007;22:229–233. doi: 10.1590/s0102-86502007000300012. [DOI] [PubMed] [Google Scholar]
- 23.Barbosa AJ. [Histological technique for intramural ganglia in thick tissue preparation (author’s transl)] Rev Bras Pesqui Med Biol. 1978;11:95–97. [PubMed] [Google Scholar]
- 24.Gabella G. Detection of cells by histochemical technique. Experientia Basel. 1969;23:218–219. doi: 10.1007/BF01899135. [DOI] [PubMed] [Google Scholar]
- 25.Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216:173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- 26.Costa M, Buffa R, Furness JB, Solcia E. Immunohistochemical localization of polypeptides in peripheral autonomic nerves using whole mount preparations. Histochemistry. 1980;65:157–165. doi: 10.1007/BF00493164. [DOI] [PubMed] [Google Scholar]
- 27.Bishop AE, Carlei F, Lee V, Trojanowski J, Marangos PJ, Dahl D, Polak JM. Combined immunostaining of neurofilaments, neuron specific enolase, GFAP and S-100. A possible means for assessing the morphological and functional status of the enteric nervous system. Histochemistry. 1985;82:93–97. doi: 10.1007/BF00502095. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Suzuki M, Endo T, Tsuji S, Daniel EE. Framework of the enteric nerve plexuses: an immunocytochemical study in the guinea pig jejunum using an antiserum to S-100 protein. Arch Histol Jpn. 1986;49:159–188. [PubMed] [Google Scholar]
- 29.Alves EP, Alves AM, Pereira RV, de Miranda Neto MH, Zanoni JN. Immunohistochemical study of vasoactive intestinal peptide (VIP) enteric neurons in diabetic rats supplemented with L-glutamine. Nutr Neurosci. 2010;13:43–51. doi: 10.1179/147683010X12611460763841. [DOI] [PubMed] [Google Scholar]
- 30.Ayres M, Ayres Jr M, Ayres DL, Dos Santos AAS. BioEstat 5.0: aplicações estatísticas nas áreas das Ciências Biológicas e Médicas. Belém: MCT; IDSM; CNPq, 2007. 364p. CD-ROM. Available from: http://www.mamiraua.org.br/pt-br/publicacoes/publicacoes/2007/livros/bioestat-50/
- 31.Schreiner M, Liesenfeld O. Small intestinal inflammation following oral infection with Toxoplasma gondii does not occur exclusively in C57BL/6 mice: review of 70 reports from the literature. Mem Inst Oswaldo Cruz. 2009;104:221–233. doi: 10.1590/s0074-02762009000200015. [DOI] [PubMed] [Google Scholar]
- 32.Shiriashi CS, Azevedo JF, Da Silva AV, Sant’Ana DMG, Araújo EJA. Análise morfométrica da parede intestinal de mucinas secretadas no Øleo de frangos infectados por Toxoplasma gondii. Cienc Rural. 2009;39:2146–2153. [Google Scholar]
- 33.Bonapaz RS, Hermes-Uliana C, Santos FN, Da Silva AV, Araújo EJA, Sant’Ana DMG. Effects of infection with Toxoplasma gondii oocysts on the intestinal wall and the myenteric plexus of chicken (Gallus gallus) Pesq Vet Bras. 2010;30:787–792. [Google Scholar]
- 34.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 35.Odorizzi L, Moreira NM, Gonçalves GF, da Silva AV, Sant’ana Dde M, Araújo EJ. Quantitative and morphometric changes of subpopulations of myenteric neurons in swines with toxoplasmosis. Auton Neurosci. 2010;155:68–72. doi: 10.1016/j.autneu.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Furness JB. The enteric nervous system. New York: Churchill Livinestone; 2006. pp. 4–280. [Google Scholar]
- 37.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 38.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002;195:1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barragan A, Sibley LD. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 2003;11:426–430. doi: 10.1016/s0966-842x(03)00205-1. [DOI] [PubMed] [Google Scholar]
- 40.da Silva JM, da Silva AV, Araújo EJ, Sant’ana Dde M. [The effects of the infection caused by Toxoplasma gondii on the cat duodenal wall] Rev Bras Parasitol Vet. 2010;19:55–61. doi: 10.4322/rbpv.01901010. [DOI] [PubMed] [Google Scholar]
- 41.Braga CF, D Da Silva AV, Sant’ana DMG, Araújo EJA. Infecção toxoplásmica causa hipertrofia da parede do cólon de frangos. Arq Bras Med Vet Zoot. 2011;63:340–347. [Google Scholar]
- 42.Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 43.Giaroni C, De Ponti F, Cosentino M, Lecchini S, Frigo G. Plasticity in the enteric nervous system. Gastroenterology. 1999;117:1438–1458. doi: 10.1016/s0016-5085(99)70295-7. [DOI] [PubMed] [Google Scholar]
- 44.Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Taché Y, Wood JD. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 45.Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81–93. doi: 10.1002/glia.10169. [DOI] [PubMed] [Google Scholar]
- 46.Anitha M, Chandrasekharan B, Salgado JR, Grouzmann E, Mwangi S, Sitaraman SV, Srinivasan S. Glial-derived neurotrophic factor modulates enteric neuronal survival and proliferation through neuropeptide Y. Gastroenterology. 2006;131:1164–1178. doi: 10.1053/j.gastro.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cirillo C, Sarnelli G, Esposito G, Grosso M, Petruzzelli R, Izzo P, Calì G, D’Armiento FP, Rocco A, Nardone G, et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol Motil. 2009;21:1209–e112. doi: 10.1111/j.1365-2982.2009.01346.x. [DOI] [PubMed] [Google Scholar]
- 48.Mourad FH, Barada KA, Bou Rached NA, Khoury CI, Saadé NE, Nassar CF. Inhibitory effect of experimental colitis on fluid absorption in rat jejunum: role of the enteric nervous system, VIP, and nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2006;290:G262–G268. doi: 10.1152/ajpgi.00271.2005. [DOI] [PubMed] [Google Scholar]
- 49.Neunlist M, Barouk J, Michel K, Just I, Oreshkova T, Schemann M, Galmiche JP. Toxin B of Clostridium difficile activates human VIP submucosal neurons, in part via an IL-1beta-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1049–G1055. doi: 10.1152/ajpgi.00487.2002. [DOI] [PubMed] [Google Scholar]
- 50.Ekblad E, Bauer AJ. Role of vasoactive intestinal peptide and inflammatory mediators in enteric neuronal plasticity. Neurogastroenterol Motil. 2004;16 Suppl 1:123–128. doi: 10.1111/j.1743-3150.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 51.Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2007;292:G231–G241. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- 52.Celikbilek A, Celikbilek M, Sabah S, Tanık N, Borekci E, Dogan S, Akin Y, Baldane S, Deniz K, Yilmaz N, et al. The Serum S100B Level as a Biomarker of Enteroglial Activation in Patients with Ulcerative Colitis. Int J Inflam. 2014;2014:986525. doi: 10.1155/2014/986525. [DOI] [PMC free article] [PubMed] [Google Scholar]


