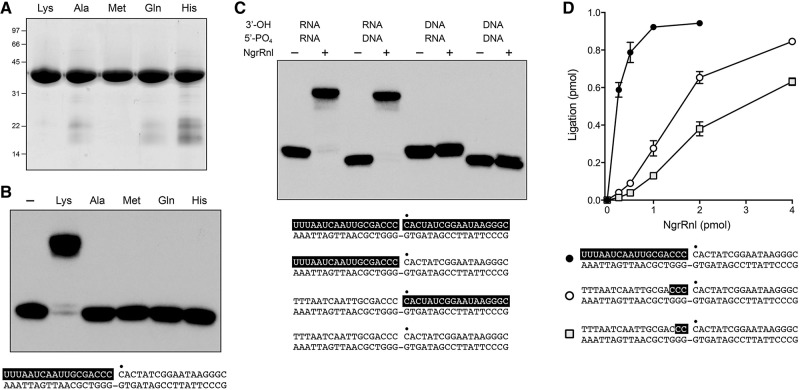
FIGURE 2.
NgrRnl is a nick-sealing RNA ligase. (A) Aliquots (10 μg) of purified recombinant wild-type NgrRnl with motif I Lys170, or mutated versions in which Lys170 was replaced by Ala, Met, Gln, or His, were analyzed by SDS-PAGE. The Coomassie blue-stained gel is shown. The positions and sizes (kDa) of marker polypeptides are indicated on the left. (B) Nick-sealing activity requires Lys170. Reaction mixtures (10 μL) containing 50 mM Tris–acetate (pH 6.0), 5 mM MnCl2, 0.2 mM ATP, 5 mM DTT, 1 pmol (0.1 μM) of 32P-labeled nicked duplex (shown at the bottom, with the 32P label at the nick denoted by • and the RNAOH strand depicted in white on a black background), and either no NgrRnl (−) or 5 pmol of NgrRnl as specified were incubated for 30 min at 37°C. The products were resolved by urea-PAGE and visualized by autoradiography. (C) Requirement for RNA on the 3′-OH side of the nick. Reaction mixtures (10 μL) containing 50 mM Tris–acetate (pH 6.0), 5 mM MnCl2, 0.2 mM ATP, 5 mM DTT, 1 pmol (0.1 μM) of 32P-labeled nicked duplex as specified (the structures of the four substrates is illustrated at the bottom, with the 32P label at the nick denoted by • and RNA strands depicted in white on a black background), and either no enzyme (−) or 5 pmol NgrRnl (+) were incubated for 30 min at 37°C. (D) Reaction mixture containing 50 mM Tris–acetate (pH 6.0), 5 mM MnCl2, 0.2 mM ATP, 5 mM DTT, 1 pmol (0.1 µM) 32P-labeled nicked duplex containing 2, 3, or 12 ribonucleotides (in white lettering on a black background), and NgrRnl as specified were incubated for 30 min at 37°C.