FIGURE 3.
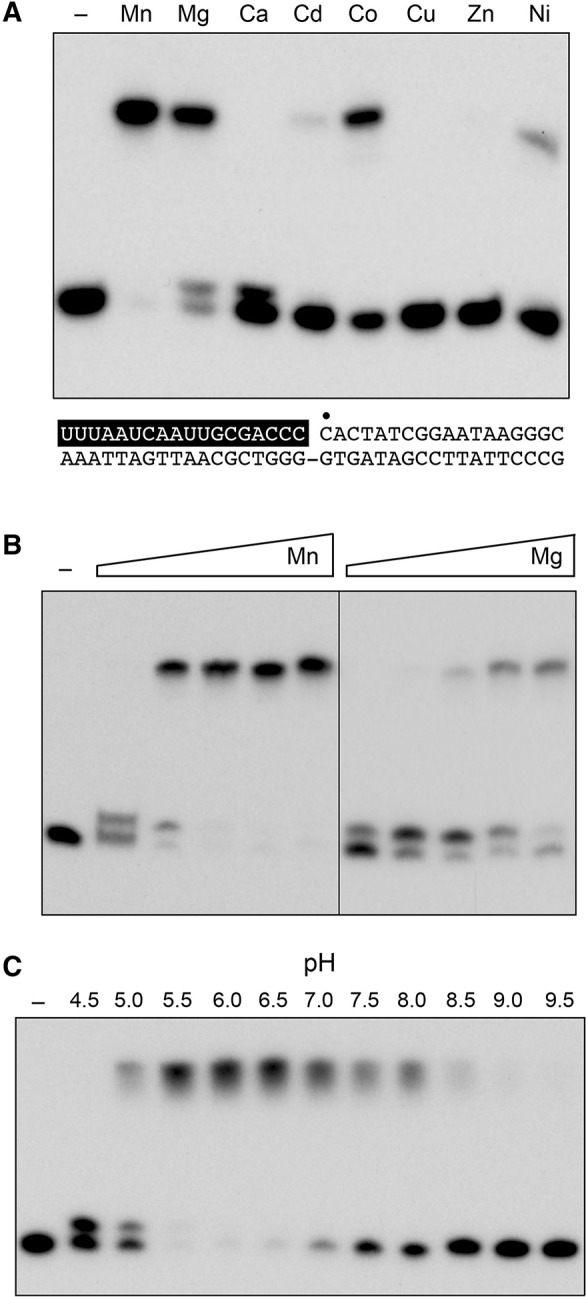
Optimal reaction conditions for nick sealing. (A) Divalent cation requirement. Reaction mixtures (10 μL) containing 50 mM Tris–acetate (pH 6.0), 0.2 mM ATP, 1 pmol (0.1 μM) of 32P-labeled nicked duplex (shown at the bottom, with the 32P label at the nick denoted by • and the RNAOH strand depicted in white on a black background), 1 pmol NgrRnl, and 5 mM of the indicated divalent cation (as the chloride salt) were incubated for 30 min at 37°C. Divalent cation was omitted from a control reaction in lane –. The products were resolved by urea-PAGE and visualized by autoradiography. (B) Divalent cation concentration dependence. Reaction mixtures (10 μL) containing 50 mM Tris–acetate (pH 6.0), 0.2 mM ATP, 1 pmol (0.1 μM) of 32P-labeled nicked duplex, 1 pmol NgrRnl, and increasing concentrations of MgCl2 or MnCl2 (0.31, 0.62, 1.25, 2.5, and 5 mM, proceeding from left to right) were incubated for 30 min at 37°C. Divalent cation was omitted from a control reaction in lane –. (C) pH profile. Reaction mixtures (10 μL) containing 50 mM Tris buffer (either Tris–acetate pH 4.5, 5.0, 5.5, 6.0, 6.5, and 7.0 or Tris–HCl pH 7.5, 8.0 8.5, 9.0, and 9.5), 5 mM MnCl2, 0.2 mM ATP, 1 pmol (0.1 μM) of 32P-labeled nicked duplex, and 1 pmol NgrRnl, were incubated for 30 min at 37°C. NgRnl was omitted from a control reaction in lane –.
