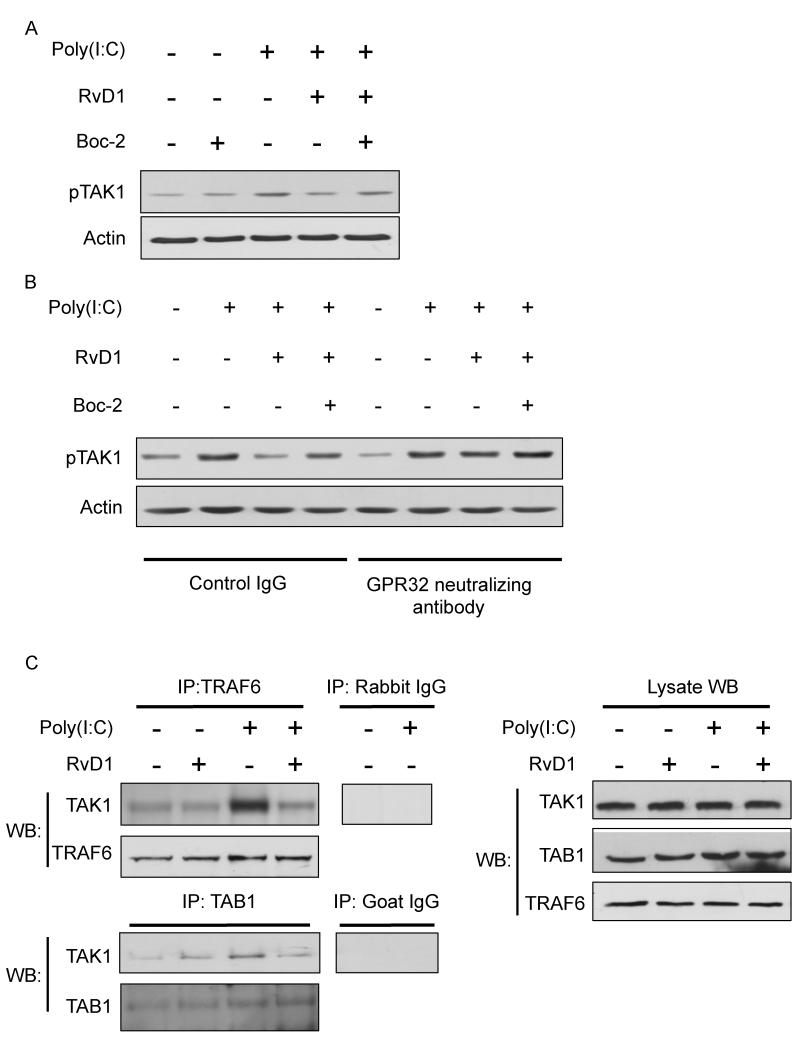
Figure 6. Blockade of FPR2/ALX and GPR32 reverses the inhibitory effect of RvD1 on poly(I:C)-induced TAK1 activation.
(A) SAECs were pretreated with Boc-2 (1μM) for 30 minutes, followed by RvD1(100nM) for a further 30 minutes. Cells were then exposed to poly(I:C) (5μg/ml) for 10 minutes to activate TAK1. Cell lysates were collected and TAK1 phosphorylation (p-TAK1) was analyzed by Western Blotting. The same membrane was stripped and re-probed using total actin as a loading control. Data shown are from the same blot from one representative experiment out of three. (B) SAECs were pre-treated with GPR32 neutralizing antibody, with or without Boc-2, for 30 minutes. Cells were then treated RvD1 (100nM) for another 30 minutes, followed by poly(I:C) for 10 minutes. Cell lysates were collected and analyzed as described previously. Data shown are from the same blot from one representative experiment out of two. Total actin was used as a loading control. (C) Cells were pretreated with RvD1 (100nM) prior to the addition of poly(I:C) for a further 10 minutes. Poly(I:C)-induced signaling complex was immunoprecipitated with antibodies against TRAF6 or TAB1 followed by Western Blotting analyses with anti-TAK1 antibody. IgG control lanes were precipitated with non-specific rabbit or goat control antibody. Lysate indicates 1/10 input in each experiment. Levels of total TAK, TAB1 and TRAF6 in each sample were determined by Western Blotting.