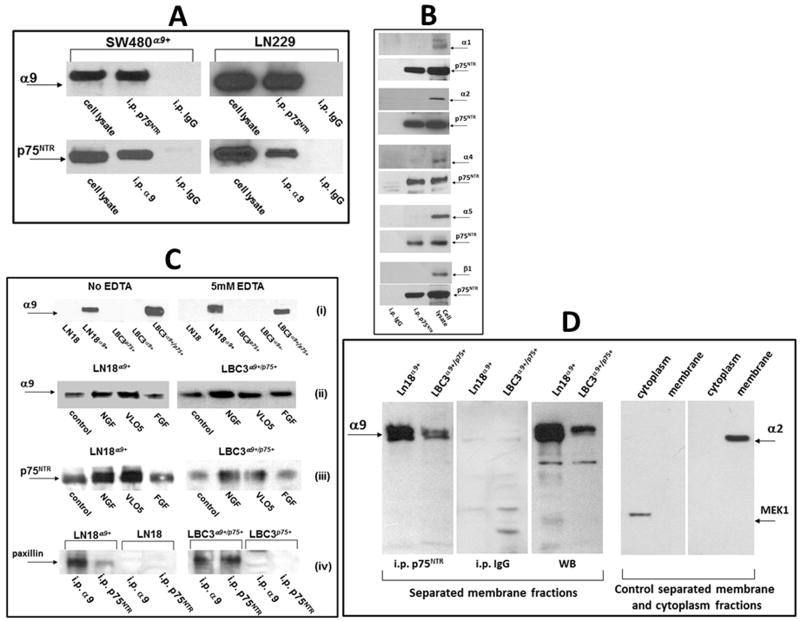
Fig. 1.
Detection of the α9/p75NTR complex in cellular lysates by immunoprecipitation. (A) Lysates of SW480α9+ and LN229 non-starved cells were immunoprecipitated with monoclonal antibodies against the indicated receptors. Detection of associated protein was performed by WB using polyclonal antibodies. Isotopic IgG for each of precipitation experiments was used as a control. (B) Lysates of LN229 cells were immunoprecipitated with anti-p75NTR mab and WB was performed with polyclonal antibodies against the indicated integrin subunits or against p75NTR. Whole lysates of LN229 cells were applied for the control detection of integrin subunits (right lines). (C) (i) Immunoprecipitation with anti-p75NTR mab from lysates of indicated cell lines in the presence or absence of EDTA; (ii) immunoprecipitation with anti-p75NTR mab or (iii) anti-α9βl mab from lysates of indicated cells previously treated or not for 60 min. with NGF (100 ng/ml), VLO5 (1μM) and bFGF (100 ng/ml), WB was performed with anti-α9 or anti-p75NTR polyclonal antibodies; (iv) immunoprecipitation with indicated mabs from cells expressing or not the α9/p75NTR complex, detection of associated paxillin was performed by WB using a polyclonal antibody. Graphic intensities of bands are presented on plots in Fig. S2. (D) Detection of the α9/p75NTR complex in separated membrane fractions by immunoprecipitation. Membrane fractions were separated from other cellular components using Subcellular Protein Fractionation Kit. Immunoprecipitation with anti-p75NTR mab or control isotopic IgG, as well as control Western blot (WB) with anti-α9 polyclonal antibody is presented in left panel. Efficiency of separation of membrane from cytoplasmic subcellular fractions was evaluated by Western blot detection of positive markers, MEK-1 for cytoplasm and α2 integrin subunit for membrane (right panels).