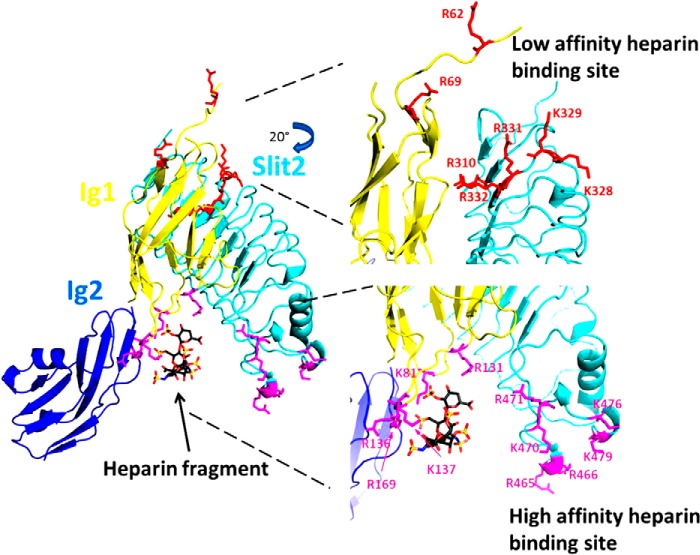
FIGURE 10.
A model of the mechanism of Slit2-Robo1-heparin interactions. The Slit2-Robo1 complex has two binding sites for heparin: the previously identified high affinity binding site near the Ig1-Ig2 interface of Robo1 (magenta) and a novel low affinity binding site located near the disordered N terminus of Robo1 as well as within adjacent conserved basic residues in Slit2 (cyan). Full-length heparin/HS binds first to the high affinity binding site, which then allows for binding of a separate portion of the heparin/HS chain to the low affinity binding site. The binding to the low affinity binding site prompts conformational changes required for signal transduction. This model was generated using the x-ray crystal structure of the second LRR domain of human Slit2 in complex with the Ig1 domain of human Robo1 (Protein Data Bank code 2V9T). The heparin tetrasaccharide and the Ig2 domain of Drosophila Robo1 were aligned and joined from the x-ray crystal structure of dRobo1 bound to heparin dp8 (Protein Data Bank code 2VRA).