FIGURE 3.
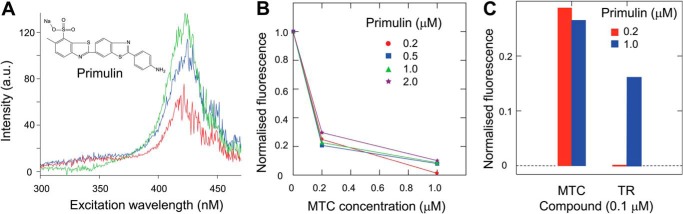
Disruption of PHFs by MT. A, PHF-dependent fluorescence excitation spectra (emission wavelength, 480 nm) measured with primulin and increasing concentrations of PHFs (2, 5, and 10 μl of PHFs, indicated by red, blue, and green profiles, respectively). The spectra shown have been corrected for signal measured in the absence of PHFs. B, PHF-dependent fluorescence at increasing concentrations of MTC in the presence of primulin at 0.2–2 μm, as indicated, with 10 μl of PHFs. The fluorescence signal measured at 420-nm excitation and 480-nm emission was corrected by subtraction of the signal measured in the absence of PHFs and normalized to the signal measured in the absence of MTC. There was no difference in MTC inhibition of fluorescence over the range of primulin concentrations tested. C, thiazine red (TR) at 0.1 μm binds to PHFs as a ligand and inhibits fluorescence seen with primulin at 0.2 μm (note the expanded vertical scale for normalized fluorescence). This inhibition can be reduced by increasing the primulin concentration to 1 μm, indicating that inhibition of primulin binding is competitive. By contrast, the inhibition of fluorescence produced by 0.1 μm MTC cannot be reduced by increasing the concentration of primulin from 0.2 to 1.0 μm, consistent with disaggregation of PHFs by MTC reported previously (10).
