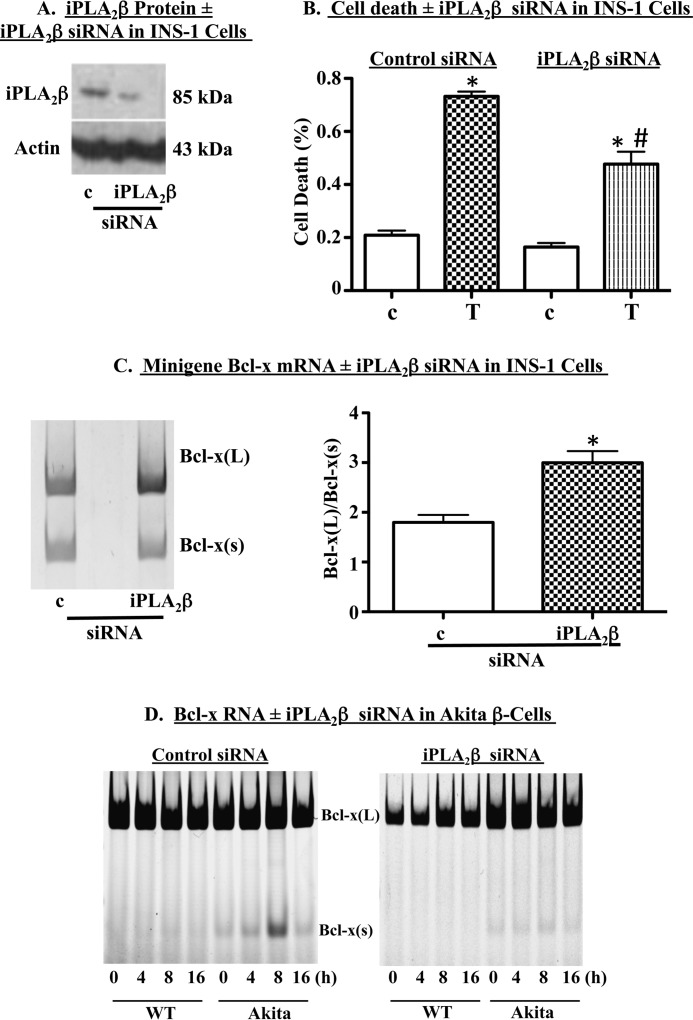
FIGURE 4.
Genetic ablation of iPLA2β promotes selection of the downstream Bcl-x 5′ SS in human Bcl-x minigene. INS-1 cells were transfected with control (c) or iPLA2β (iPLA2β) siRNA. A, representative immunoblot analysis of iPLA2β protein in transfected cells. B, INS-1 cells were transfected with control or iPLA2β siRNA and then treated with DMSO (c) or 1 μm thapsigargin (T). Cell death was quantified through trypan blue exclusion assays. Shown are mean ± S.E. from four independent experiments. (*, T group significantly different from control-c or iPLA2β-c, p < 0.0001; #, iPLA2β-T group significantly different from control T group, p < 0.0001.) C, INS-1 cells were co-transfected with Bcl-x minigene and c- or iPLA2β-siRNA. Cells were cultured for 13 h, and then RNA was harvested and RT-PCR performed to amplify minigene splice variants. Shown are a representative experiment (left panel) and quantification (right panel) of four independent experiments (mean ± S.E.). (*, significantly different from control siRNA treatment group, p < 0.05.) D, wild-type (WT) and Akita β-cells were transfected with control (left)- or iPLA2β (right)-siRNA and then treated with 1 μm thapsigargin for 4–16 h. RNA was extracted and RT-PCR performed to amplify Bcl-x splice variants. A representative experiment is shown. Each representative experiment was performed at least twice.