FIGURE 7.
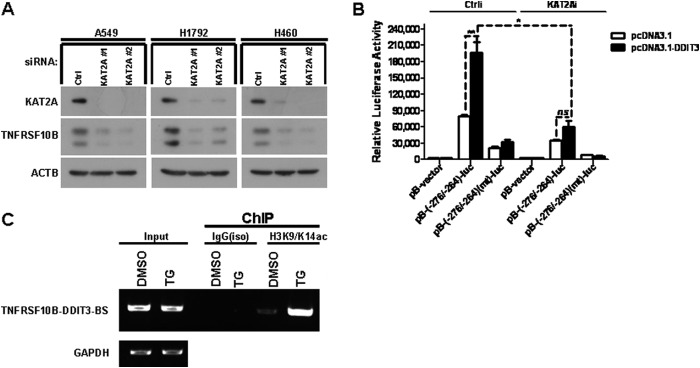
KAT2A is a co-activator of DDIT3 and enhances TNFRSF10B transcription by mediating acetylation of H3K9/K14. A, A549, H1792, and H460 cells were transfected with KAT2A siRNAs (#1 and #2) for 24 h, and samples were analyzed by Western blot using antibodies against KAT2A, TNFRSF10B, and ACTB. Ctrl, control. B, H1792 cells were co-transfected with KAT2A siRNA and pB-TNFRSF10B/DDIT3(−276/−264)-BS-luc (0.25 μg/well) or pB-TNFRSF10B/DDIT3(−276/−264)(mt)-BS-luc (0.25 μg/well) for 24 h. Cell lysates were prepared, and luciferase assays were performed according to the manufacturer's instructions. The transfection efficiency was normalized by co-transfection with pCH110 (β-gal) (0.1 μg/well). All luciferase activity detection experiments were performed three times independently, and the values obtained were used to calculate means and standard deviations. C, H1792 cells were treated with DMSO and TG (1 μm) for 12 h, and then the cell lysates were prepared and ChIP assays were performed using the anti-H3K9/K14ac antibody and the normal IgG antibody as negative control. The input and immunoprecipitated samples were used as template for PCR amplification of fragments containing the DDIT3 binding site, and GAPDH was used as a loading control. Columns, mean of triplicate treatments; bars, ± S.D. The statistical differences between the two treatments were analyzed by two-sided unpaired Student's t tests (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, no significant difference).
