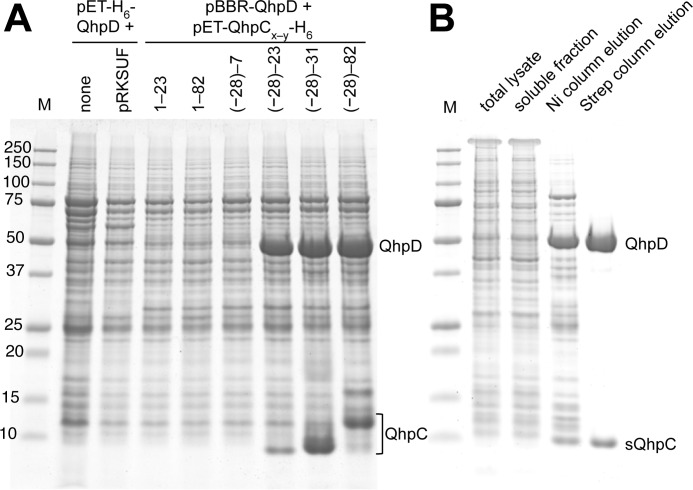
FIGURE 4.
Interaction of QhpD with various forms of QhpC and affinity purification of the QhpC·QhpD complex. A, E. coli cells transformed with either pET-H6-QhpD alone, pET-H6-QhpD plus pRKSUF, or pBBR-QhpD plus one of the pET-QhpC-H6 plasmids (encoding various lengths of QhpC as indicated in the figure) were grown in 50 ml of LB medium containing appropriate antibiotics, 0.17 mg/ml of ammonium ferric citrate, and 0.1 mm isopropyl β-d-thiogalactopyranoside, and disrupted by sonication in 50 mm potassium phosphate buffer, pH 7.8, containing 300 mm NaCl. Cell extracts (2.4 ml) were applied to nickel-nitrilotriacetic acid spin columns (Qiagen) in four load-and-spin cycles, and eluted proteins were precipitated with 10% (w/v) TCA and dissolved in 50 μl of loading buffer for SDS-PAGE. A 10-μl aliquot of the resultant solution was loaded in each lane. B, E. coli cells harboring pET-H6-QhpD and pBBR-sQhpC-St2 were disrupted by sonication (total lysate). The cell extract obtained by centrifugation (soluble fraction) was purified first by a nickel chelate column (Ni column elution) and second by a Strep-Tactin column (Strep column elution). A total of 7 μg of protein was loaded in each lane. The protein bands were stained with colloidal Coomassie G-250 (54).