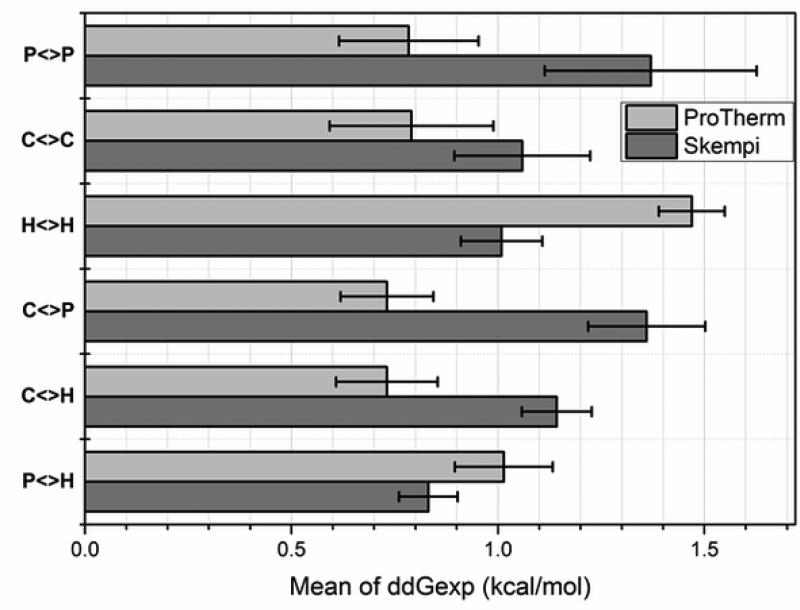
Fig. 6.
The mean of the experimental folding and binding free energy change caused by mutations taken from ProTherm and Skempi databases and grouped by physico-chemical property as: P<>P – polar to polar side chain substitution; C<>C – charged to charged; H<>H – hydrophobic to hydrophobic, C<>P – charged to polar and polar to charged; C<>H – charged to hydrophobic and hydrophobic to charged; P<>H – polar to hydrophobic and hydrophobic to polar. Charged residues (C): R, K, D and E; polar residues (P): S, T, N, and Q; hydrophobic residues (H): A, V, I, and L. Standard error is provided for each bar.