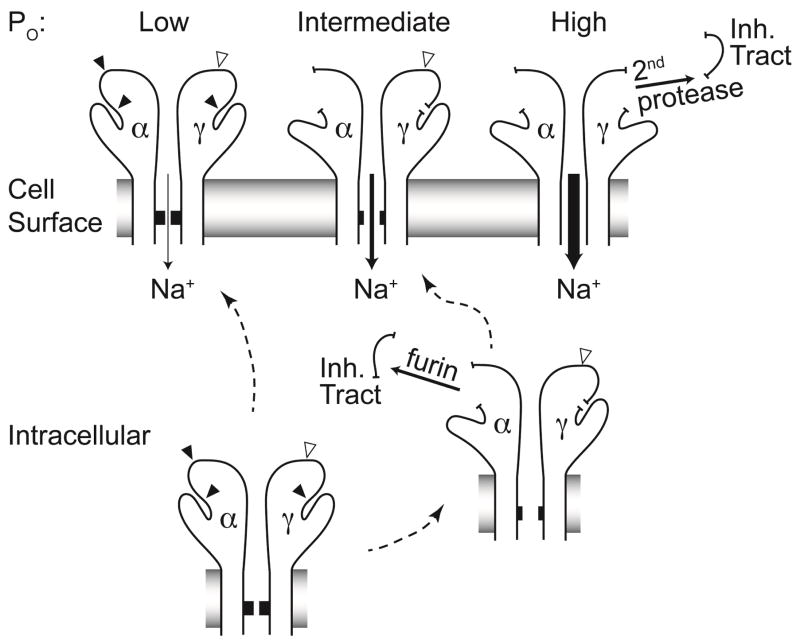
Figure 1.
Two of ENaC’s three subunits (α and γ) undergo proteolytic cleavage. Furin cleaves the channel before it reaches the cell surface (closed arrowheads). Some channels escape furin cleavage and arrive at the cell surface intact. These channels exhibit very low activity, or open probability (Po). Furin cleaves the α subunit twice and the γ subunit once. Dual cleavage of the α subunit by furin releases that subunit’s inhibitory tract, resulting in channels with intermediate activity. The γ subunit harbors one furin cleavage site proximal to its inhibitory tract, and sites for various other extracellular proteases distal to the inhibitory tract (open arrowhead). Release of the γ subunit inhibitory tract occurs when the subunit has been cleaved by both furin and a second protease, resulting in a maximally activated channel.