Figure 2.
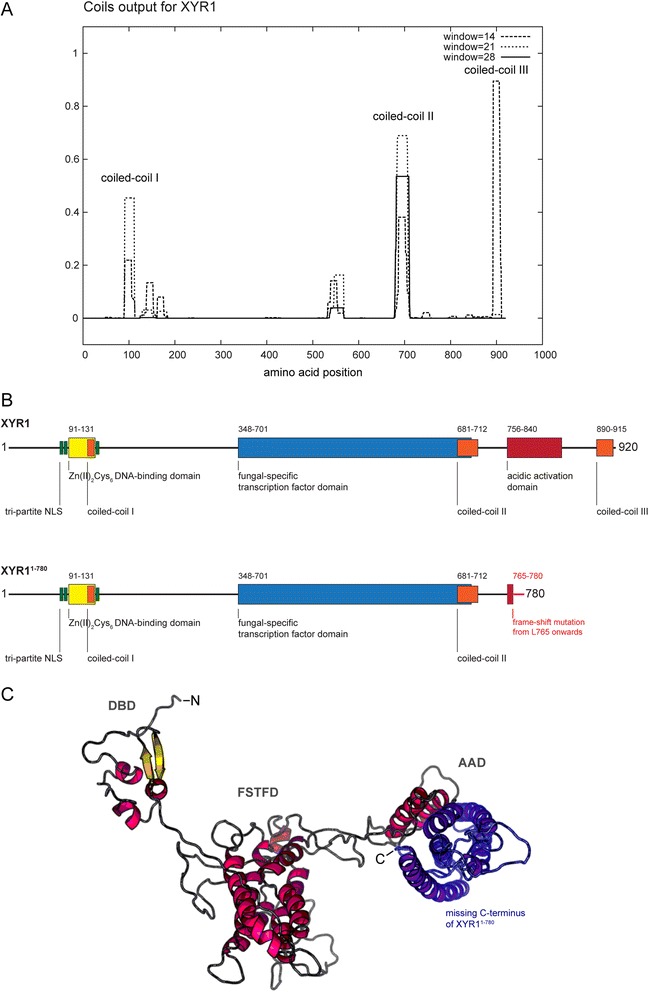
XYR1 structure and structural consequences of the truncated XYR1–780. (A) Predicted coiled-coil domains in XYR1 using COILS, which compares a sequence to a database of known parallel two-stranded coiled-coils and derives a similarity score from which the probability that the sequence will adopt a coiled-coil confirmation is calculated. (B) XYR1 contains a classical tri-partite nuclear localisation signal (NLS; green) which directs the transcription factor into the nucleus. XYR1’s DNA-binding domain (DBD; yellow) is orthologous to that of Gal4 from Saccharomyces cerevisiae. The first coiled-coil domain of XYR1 (orange) is located at the end of the DBD. The filamentous fungal-specific transcription factor domain (FSTFD; blue) regulates target gene expression and constitutes the biggest part of the protein. A second coiled-coil domain (orange) overlaps with the C-terminal end of the FSTFD. The acidic activation domain (AAD; dark red) is a characteristic feature of Zn2Cys6-type transcription factors. The third coiled-coil domain (orange) sits at the very C-terminus, and likely mediates homodimerisation of XYR1. Hence, the truncated XYR11–780 protein encoded in QM9136 almost completely lacks the AAD and fully lacks the third coiled-coil domain due to a frame-shift mutation from L765 onwards. The terminal 16 amino acids (L765-F780; light red) encode a non-sense sequence that finds no BLASTp hit in the T. reesei genome. (C) 3D-reconstruction of XYR1. RaptorX protein structure prediction software (raptorx.uchicago.edu) identifies three functional domains in XYR1, resembling DNA-binding domain (DBD), fungal-specific transcription factor domain (FSTFD) and acidic-activation domain (AAD), perfectly matching our manual annotation (B). Five of the seven α-helices comprising the AAD are missing in the truncated XYR11–780 protein (highlighted in blue). The closest structural orthologs are the Zn2Cys6-type transcriptional regulators Ppr1 and Gal4 from S. cerevisiae, both known to be active as non-symmetrical homodimers [63].
