Abstract
Aberrant sialylation in glycoproteins and glycolipids is a characteristic feature of malignancy. Human sialidases, which catalyze the removal of sialic acid residues from glycoconjugates, have been implicated in cancer progression. They have been detected in a wide variety of human cells and tissues, but few studies have focused on their existence in human serum. Among the four types identified to date, we previously demonstrated that plasma membrane-associated ganglioside sialidase (NEU3) is markedly upregulated in various human cancers, including examples in the colon and prostate. Here, using a sensitive assay method, we found a significant increase of sialidase activity in the serum of patients with prostate cancer compared with that in healthy subjects having low activity, if any. Activity was apparent with gangliosides as substrates, but only to a very limited extent with 4-methylumbelliferyl sialic acid, a good synthetic substrate for sialidases other than human NEU3. The serum sialidase was also almost entirely immunoprecipitated with anti-NEU3 antibody, but not with antibodies for other sialidases. Interestingly, sera additionally contained inhibitory activity against the sialidase and also against recombinant human NEU3. The sialidase and inhibitor activities could be separated by exosome isolation and by hydrophobic column chromatography. The serum sialidase was assessed by a sandwich ELISA method using two anti-NEU3 antibodies. The results provide strong evidence that the serum sialidase is, in fact, NEU3, and this subtype may, therefore, be a potential utility for novel diagnosis of human cancers.
Keywords: Cancer serum, diagnostic marker, ganglioside, prostate cancer, sialidase
Alterations in glycosylation occur during tumorigenesis, and aberrant sialylation in particular has been implicated in the malignant phenotype with reference to metastatic potential and invasiveness.1,2 Mammalian sialidases are the key enzymes controlling cellular sialic acid contents, through catalyzing the initial step of degradation of glycoproteins and glycolipids by removing sialic acid residues from their carbohydrate portions. Four types of human sialidases have been identified to date, designated as NEU1, NEU2, NEU3 and NEU4, and characterized as differing in major subcellular localization and enzymatic properties, including substrate specificity, as well as in chromosomal sites.3 They appear to behave in different independent manners during carcinogenesis.4 NEU1, NEU3 and NEU4 have been detected in various cells and tissues as major human sialidases,5,6 but no details on serum sialidase have been reported, except for the activity toward 4-methylumbelliferyl sialic acid (4MU-NeuAc) as a substrate, found to be associated with immunoglobulin IgG in the serum of multiple myeloma patients.7
Among the four human sialidases, the plasma membrane-associated sialidase, NEU3, is unique in substrate preference for gangliosides, co-existing at the cell surface, therefore being suggested to participate in transmembrane signaling.8 NEU3 is markedly upregulated in various human cancers, including colon,9 renal,10 ovarian11 and prostate12, and contributes to augmentation of malignant properties of cancer cells, probably by causing disturbance of transmembrane signaling. In contrast, NEU113 and NEU414 may be downregulated in colon cancer. In the present study, we found ganglioside sialidase activity detectable in the serum of prostate cancer patients applying a sensitive method with fluorometric high-performance liquid chromatography (HPLC) using malononitrile,15 with significant elevation as compared to normal subjects. We obtained evidence of NEU3 identity for the sialidase, and additionally, demonstrated the presence of inhibitory activity for the sialidase. Although the functional significance and the mechanisms of appearance in serum remain to be elucidated, this is the first report describing the existence of serum sialidase of human origin and increase in cancer patients, providing a pointer to a new diagnostic tool.
Materials and Methods
Serum samples
Human sera were obtained from 3 female and 10 male healthy adults, and from 34 patients diagnosed with prostate cancer at various clinical stages in the Urology Department of Miyagi Cancer Center. Non-hemolytic blood samples, drawn from the cubital vein, were allowed to coagulate at room temperature for 30 min and were subsequently centrifuged at 2000 g for 10 min. Supernatants were carefully collected to avoid contamination by blood cells. Informed consent was obtained from each subject to allow use of the blood for research purposes, and the study was approved by the Committee on Human Rights in Research at Miyagi Cancer Center.
Sialidase activity assays
Human sera were used for sialidase assays using gangliosides GM3 (NeuAc α2-3Galβ1-4Glcβ1-1Cer) (Alexis Biochemicals, Lansen, Switzerland) or 4MU-NeuAc as the routine substrates in 25 μL of reaction mixture. Activities for NEU1, NEU2 and NEU4 were measured with mixtures containing 2.5 μmol sodium acetate buffer (pH 4.6 for NEU1 and NEU4; pH 5.5 for NEU2), 10 μg bovine serum albumin (BSA) and 20 nmol 4MU-NeuAc. After incubation for 30 to 60 min at 37°C, the 4-methylumbelliferone released was determined fluorometrically.15 The reaction mixture for assays of NEU3 activity contained 2.5 μmol sodium acetate buffer (pH 4.5), 10 μg BSA, 5 nmol GM3 and 25 μg Triton X-100. The sialic acids released from GM3 were measured by fluorometric HPLC with malononitrile.15 On occasion, NEU4 activity was also assayed with GM3 as the substrate in the same manner, because of its potential hydrolytic ability toward GM3 as well as 4MU-NeuAc. Protein concentrations were determined by dye-binding assay (Bio-Rad Laboratories, Hercules, CA, USA). One unit was defined as the amount of enzyme cleaving 1 nmol of sialic acid/h.
Preparation of recombinant sialidases
HK-293 T cells (2∼5 × 107) were transfected with FLAG-tagged sialidase cDNA using Effectene reagent (QIAGEN). At 48 h after transfection, the cells were collected, washed with PBS, and sonicated on ice in 9 volume of ice-cold lysis buffer. The lysates were centrifuged at 1000 g for 10 min at 4°C and the resultant supernatants (homogenates) were then used for measurement of sialidase activity. For the NEU1 enzyme, a cDNA for a protective protein (carboxypeptidase A), which is known to be associated with NEU1 protein and β-galactosidase as a complex in the lysosomes to maintain the sialidase activity,16 was co-transfected. The lysis buffer for NEU1 and NEU2 contained 20 mM potassium phosphate pH 6.8, 0.15 M NaCl, 1 mM phenymethylsulfonyl fluoride (PMSF), and Protease Inhibitor Cocktail (Roche, Basel, Switzerland), while that for NEU3 and NEU4 contained 1 mM EDTA and 1% Triton X-100. The detailed procedures were as described previously.5
Preparation of antibodies
Anti-human NEU1 antibody was prepared by immunizing rabbits with keyhole limpet hemocyanin-conjugated oligopeptides corresponding to amino acid residues 201–216 and 392–410 of human NEU1. Anti-rat NEU2 polyclonal antibody (cross-reactive with human recombinant NEU2) was obtained as described earlier.17 Anti-human NEU3 monoclonal antibody was prepared as detailed previously.18 Preparation of anti-human NEU4 antibody was accomplished by immunizing rabbits with keyhole limpet hemocyanin-coupled oligopeptides (amino acid residues 347–363) of human NEU4. Immunoprecipitation studies with serum sialidase were performed using the respective sialidase antibodies. Serum (2.5–5.0 μL) or recombinant sialidase protein (0.9–1.2 units) was immunoprecipitated with anti-sialidase antibody at 4°C for 4 h, and then with protein A Sepharose beads (GE Healthcare Life Sciences, Little Chalfont,UK) for 2 h. The immunocomplexes were then centrifuged and the resulting supernatant was assayed for sialidase activity.
Inhibitor activity determination
Serum was fractionated with ammonium sulfate, and the fraction precipitating between 50 and 80% of saturation was collected by centrifugation, and dialyzed. Serum (5.0 μL) or the dialyzed fraction of the ammonium precipitates was routinely used as the inhibitor source. Alternatively, serum was fractionated by ExoQuick (System Biosciences, Mountain View, CA, USA) according to the manufacturer's recommended procedure, and the resulting exosome fractions were also used as a sialidase inhibitor source. Serum, ammonium sulfate precipitates or exosome fractions were determined for sialidase inhibitory activity by incubating with recombinant sialidase (0.9–1.2 units) and substrate for 30–60 min in the same assay system as that for sialidase activity. The inhibitory activity was defined as the percentage of residual sialidase activity relative to the control value without inhibitor.
ELISA for NEU3 sialidase
To measure NEU3 protein concentrations in human serum, a sandwich ELISA method was developed using two anti-NEU3 monoclonal antibodies prepared previously.18 Serum (10 μL) was added to each well of anti-NEU3 (clone 7) antibody-coated plates (1:100 dilution with 1% BSA in PBS), which were then incubated overnight at 4°C. After washing three times with PBS-T (PBS containing 0.05% Tween20), peroxidase-conjugated anti-NEU3 (clone 11) antibody was added to each well, and the plates were incubated at room temperature for 1 h. After washing three times with PBS-T, and following treatment with TMB reagent (Sigma-aldrich, St. Louis, MO) for 10 min at room temperature, the reaction was stopped by the addition of 1 N H2SO4. Color intensity was determined at 450 nm.
Statistical analysis
The results are expressed as mean ± SD. Values were compared using Student's t-test.
Results
Identification of ganglioside sialidase acitivity in serum of prostate cancer patients with significantly higher levels than in healthy subjects
We previously demonstrated marked increase in sialidase NEU3 expression in tumor specimens of prostate cancer compared with non-tumor prostate tissues with reference to mRNA levels and immunohistochemistry staining.12 Human NEU3 has been found to be almost specific for gangliosides with hardly any action on glycoproteins, glycopeptides, or 4MU-NeuAc that are considered as good substrates for other sialidases in in vitro activity assays.19,20 Based on previous results, our attempts were made to measure the sialidase activity in the serum of prostate cancer patients. For the present assays with 2.5 μL of serum, ganglioside GM3 was first used as the substrate. As shown in Figure1(a), sialidase activity was evident in serum of prostate cancer patients, even though the level was extremely low (approximately 1/20–1/50) compared with those of prostate cancer tissues per milligram of protein. When compared with healthy subjects, however, sera of the patients showed significantly higher levels of sialidase activity toward GM3 (Fig.1b). Although our previous results demonstrated that NEU3 mRNA levels in prostate cancer tissues positively correlated with cancer grades (e.g. Gleason score [P < 0.001]) and, furthermore, immunohistochemistry with anti-NEU3 monoclonal antibody exhibited strong positive staining in the cancer tissues with a high score,12 the serum sialidase activities toward GM3 showed no statistically significant correlation with the cancer grade in the present study.
Fig 1.
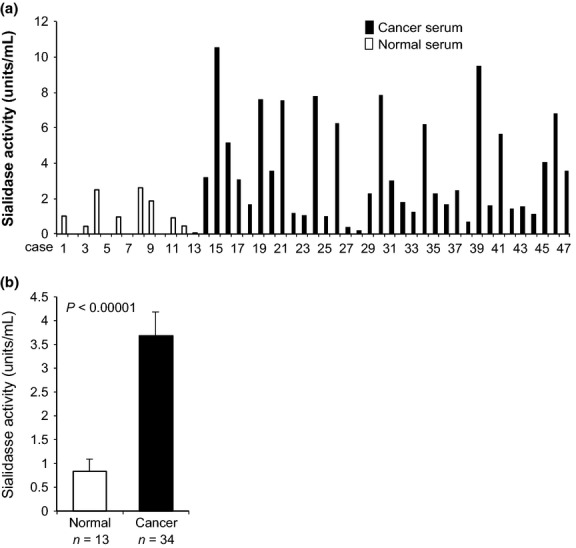
Determination of ganglioside sialidase activity in human serum and increase in the serum of prostate cancer patients. (a) Note significantly higher levels of sialidase activity toward the ganglioside GM3 substrate in serum of prostate cancer patients than in normal subjects. (b) Statistical significance of increased sialidase activity in the serum of prostate cancer patients compared with normal subjects.
Characterization of serum sialidase activity
To facilitate optimum activity for the sialidase, influence of various times of incubation and serum volumes was assessed in the assays with GM3 as the substrate. As shown in Figure2(a), linearity in activity up to 2.5 μL was obtained, but a dramatic decrease in the activity at over 5 μL serum, and especially the 80–90% reduction with 10 μL, suggested the existence of some components for activity inhibition or degradation of released sialic acids. The inhibitory component(s) will be described in the next section. The time course of the sialidase activity with 2.5 μL serum was confirmed to be linear for up to 60 min.
Fig 2.

Characterization of a ganglioside sialidase activity in human serum. (a) Sialidase activity toward GM3 gangliosides with various serum amounts. Note that with 5 μL of serum decreased sialidase activity was observed. (b) Effect of pH on the sialidase activity. Sialidase activitity was assayed using GM3 substrate in sodium acetate buffer at pH 3.5–5.5 and sodium phosphate buffer at pH 5.5–7.0. (c) Substrate specificity of the serum sialidase. Various natural substrates and a synthetic substrate, 4MU-NeuAc, were examined. (d) Immunoprecipitation studies of the serum sialidase using anti-NEU3 and NEU4 antibodies. Anti-NEU3 and anti-NEU4 antibodies precipitated recombinant NEU3 (left panel) and NEU4 (middle), respectively. The serum sialidase was precipitated only with anti-NEU3 antibody (right panel).
First, using 2.5 μL serum as an enzyme source, we examined which type of sialidase was responsible for the activity. Regarding substrate specificity, 4MU-NeuAc is capable of being used as a substrate to determine NEU1, NEU2 and NEU4 activities, and as a ganglioside for NEU3 and NEU4 in the presence of Triton X-100, although NEU3 and NEU4 cannot be clearly distinguished because of both acting on gangliosides at acidic pH. As shown in Figure2(b), with GM3 as the substrate, the pH dependence of the serum silaidase showed two peaks in the pH curve, a large one at pH 4.5–4.8 and with a smaller one at pH 5.8–6.0, similar to the case with human NEU3.20 When the substrate specificity was investigated with various substrates, the serum sialidase hydrolyzed GD3 and GM3 efficiently but there was only a little activity on oligosaccharides such as sialyllactose, glycoproteins including fetuin and 4MU-NeuAc (Fig.2c), as would be expected with NEU3.20 To obtain the results showing a strong possibility of NEU3 identity, we purified the enzyme from pooled sera (60 mL) of cancer patients with affinity chromatography immobilized anti-NEU3 antibodies followed by gel electrophoresis and liquid chromatography coupled to mass spectrometry for analyses of the protein obtained. However, the attempts failed probably due to insufficient volume of the serum as the enzyme source. Studies were then undertaken to address the issues by immunoprecipitation studies using anti-sialidase antibodies. Because the results for the substrate specificity suggested the serum sialidase to be a ganglioside-hydrolyzing sialidase, as described above, anti-NEU3 or anti-NEU4 antibodies were employed. As references, recombinant sialidases NEU3 and NEU4 were applied to verify that the antibodies could immunoprecipitate their respective sialidases. Figure2(d) (left and middle) clearly shows that the antibodies cross-react and immunoprecipitate the corresponding recombinant sialidases, whereas no mutual reactions occurred between the antibodies. Under the conditions used, the serum sialidase was evidently precipitated with only the anti-NEU3 antibody (Fig.2d, right panel). It should be noted that no significant precipitation of the serum sialidase occurred with anti-NEU1 or NEU2 antibodies (data not shown). These results together indicate that the serum sialidase should, indeed, be considered as NEU3.
Characterization of sialidase inhibitor activity in serum
Next, we investigated the properties of the inhibitor activity against sialidase. When whole serum (5 μL) of the prostate cancer patients was incubated with NEU3 or NEU4 recombinant sialidase using GM3 as substrate, the serum was found to suppress only NEU3 recombinant enzyme activity. The inhibitory activity was then fractionated in precipitates of 0–50 and 50–80% ammonium sulfate, and was detected mainly in the latter fractions (Fig.3a). To determine specificity for any other type of sialidase, the 50–80% ammonium sulfate fractions were incubated with equal amount of each recombinant sialidases with ganglioside GM3 (Fig.3b, left) or 4MU-NeuAc (Fig.3b, right) as substrates at an appropriate pH. NEU4 was tested with GM3 and 4MU-NeuAc substrates, based on its substrate preference. The results clearly confirm that the inhibition occurred specifically with NEU3, and not other sialidases, even the ganglioside sialidase NEU4. The inhibitory activity was stable after boiling for 2 min, and not affected by proteases inhibitors, including PMSF, leupeptin, pepstatin and aprotinin. Furthermore, the fraction did not contain measurable activity of N-acetylneuraminic aldolase, a degradation enzyme for released sialic acids. To exclude the possibility of albumin itself, because of its enrichment in 50–80% ammonium sulfate fraction, and of production of auto-antibodies to NEU3 protein, immunoprecipitation studies with anti-human albumin antibody and protein L-Sepharose (binding to all human immunoglobulins, Fab and k light chains), respectively, were performed by pre-incubating with the inhibitor fractions (Fig.3c,d). However, the inhibitor activity was not suppressed by these treatments. When assessed in the serum of healthy adults in the same way as for cancer serum, it was detected to a similar extent. Unlike the case with the sialidase activity, the inhibitor activity did not show a statistically significant difference between the values of cancer and healthy subjects. However, interestingly, there was a negative correlation between the sialidase and inhibitory activities (correlation coefficient, r = −0.58) only in serum of the cancer patients. Hence, it seems likely that serum of cancer patients with relatively low sialidase activity tends to contain higher inhibitory activity than the case with high sialidase activity, even though the serum (2.5 μL) used for sialidase activity assays did not exhibit apparent inhibitory activity. The molecular basis of the correlation of the two activities is unclear at present.
Fig 3.
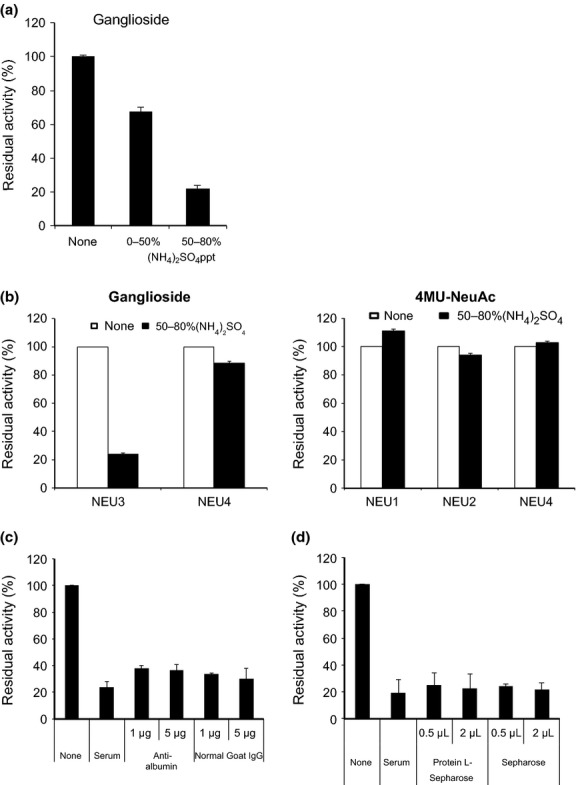
Characterization of the inhibitor activity in human serum. (a) Inhibitory effects of human serum on ganglioside sialidase activity. The inhibitory activity was defined as the percentage of residual sialidase activity relative to the controls without inhibitor. Ammonium sulfate fractions of whole serum were examined for inhibitory activity against recombinant NEU3 or NEU4 sialidase activity (0.8–1.2 units of each) with the GM3 substrate. The results here were obtained only with NEU3 but not with NEU4. (b) The 50–80% ammonium sulfate fractions were tested for inhibitory activity against recombinant sialidase (0.9–1.3 units of each) NEU3 or NEU4 with ganglioside GM3 (left panel), and NEU1, NEU2 and NEU4 with 4MU-NeuAc as the substrate (right panel). (c, d) Immunoprecipitation studies of the serum inhibitory activity with anti-human albumin goat antibody (Bethyl Laboratories, Montgomery) (c) or protein L-Sepharose (1.5 × 3 cm, BioVision Inco.) (d). As controls, goat IgG and Sepharose beads were used instead of anti-albumin antibodies and protein L-Sepharose, respectively. Recombinant NEU3 (1.5 units) was preincubated with human serum (5 μL) or serum treated with anti-albumin antibodies (c) or protein L-Sepharose (d), and each supernatant after immunoprecipitation was assayed for sialidase activity with GM3 as the substrate.
Separation of sialidase and inhibitory activities
To further characterize the two activities, separation by exosome isolation kit and hydrophobic chromatography was undertaken. Exosome isolation yielded most of the ganglioside sialidase activity in the supernatant and, in contrast, the inhibitory activity in the pellet (exosome fraction), in line with distribution pattern of an exosome marker, HSP70 (Fig.4a). These results also suggest that sialidase activity assays with 2.5 μL serum would be appropriate for obtaining the correct sialidase activity, because the value in the same volume of serum was similar to that in the supernatant when the inhibitor activity was removed by exosome isolation. To separate the two activities by column chromatography, the 40–80% ammonium sulfate fractions of cancer patient serum (3 mL), containing sialidase and inhibitory activities, were loaded on Octyl-Sepharose (1.5 × 3 cm) (Fig.4b). The sialidase activity was detected in the pass-through fractions, and the inhibitory activity was eluted with 0.2% Triton X-100, indicating that they are different proteins distinguished by differing hydrophobic properties. When each of the activity fractions from the column were concentrated and separately applied to Toyopearl HW-55F column (1.5 × 45 cm; Tosoh Bioscience Tokyo, Japan), the sialidase activity emerged a little behind the position of the BSA marker, with an apparent molecular weight of 58 KDa (Fig.4c), which is similar to that for recombinant NEU3, and inhibitor activity was detected at the position similar to BSA, with an apparent molecular weight of 65–70 kDa (Fig.4d).
Fig 4.

Separation of the sialidase from the inhibitory activities. (a) Separation of the two activities by exosome isolation kit (ExoQuick, System Biociences). Resulting supernatants after exosome isolation retained most of the sialidase activity, whereas the exosome fraction had enriched inhibitory activity. The western blot with anti-HSP70 antibody is shown for each exosome fraction to verify the fractionation. (b) Separation of the two activities on Octyl-Sepharose chromatography. Ammonium sulfate fractions (40–80%, containing sialidase and inhibitory activities) of cancer patient serum (3 mL) were dissolved in 0.1 M phosphate buffer (pH 6.8) containing 0.5 M NaCl and 0.2 mM PMSF, and loaded on a Octyl-Sepharose column (1.5 × 3 cm, GE health care) equilibrated with the same buffer. After washing the column with the same buffer, the sialidase activity was eluted with 10 mM phosphate buffer containing 0.2 mM PMSF, and then the inhibitory activity followed with 0.2% Triton X-100 in the buffer. (c,d) The each activity fractions from the Octyl-Sepharose column were concentrated and separately applied to the Toyopearl HW-55F column (1.5 × 45 cm, Tosoh Bioscience) to estimate the apparent molecular size. BSA, bovine serum albumin.
Quantification of serum NEU3 by sandwich ELISA
For the purpose of quantitation of serum NEU3, sandwich ELISA was performed using two anti-NEU3 monoclonal antibodies prepared previously. The standard curve showed linearity at the 0.1 to 10 μg protein concentrations of the homogenates of NEU3-transfected HK-293T as a standard (Fig.5a). Under the conditions adopted, the serum NEU3 concentrations of each prostate cancer patient and normal adult were calculated. As with sialidase activity shown in Figure1(b), the ELISA tests showed the levels of NEU3 in serum of prostate cancer patients to be higher than those in normal subjects with statistical relevance (Fig.5b), validating the results of sialidase activity assays.
Fig 5.
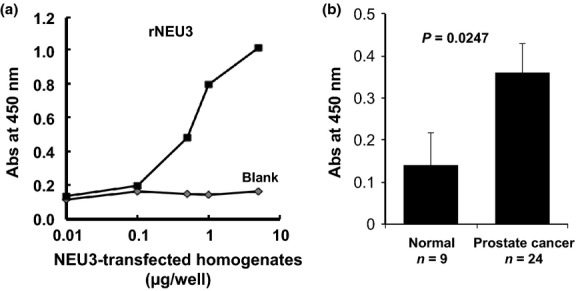
Sialidase NEU3 assay by ELISA. (a) Standard curve of sialidase NEU3 concentrations using recombinant NEU3 protein in the sandwich ELISA. Homogenates of vector-transfected and NEU3-transfected 293T cells were used as the control and NEU3 standard, respectively. (b) The results of increased sialidase NEU3 in the serum of prostate cancer patients (n = 24) compared with normal sera (n = 9) as assessed by the ELISA method.
Discussion
We previously demonstrated remarkable upregulation of sialidase NEU3 in various human cancers, including prostate cancer, showing an apparent acceleration of tumor progression mostly by disturbance of cellular signaling at the cell surface.4 Based on these results, the aim of the present study was to investigate whether NEU3 could be detected in the serum of cancer patients. We focused on prostate cancer for development of a new diagnosis marker, because measurement of serum PSA as the most frequently used tool is often associated with substantial false-positives.21 Although our previous results on prostate cancer suggested that the NEU3 expression level in tissue specimens could be a more sensitive marker than PSA for early diagnosis to discriminate non-cancer from malignant prostate lesions,12 it is definitely preferable to use a non-invasive diagnostic method. As was hoped, sialidase activity considered due to NEU3 was identified in the serum with significantly higher levels than in normal subjects. This is the first evidence for the existence of a mammalian sialidase in serum.
At present, the functional significance of the sialidase and the reason for its appearance in serum are uncertain, but it may be associated with the existence of gangliosides in serum. There have been reports of gangliosides in human serum,22–24 and it has been suggested that their shedding by some tumor cells may occur at strikingly high rates, correlated with tumorigenicity and cell density.22 Serum gangliosides in cancer patients have been described to undergo pronounced changes with relatively high amounts, probably contributing to the suppression of the immune system that is frequently evident in tumor-bearing hosts.24 To compensate for the high amounts of gangliosides in serum of cancer patients, NEU3 may be secreted from the cell surface. Alternatively, it could be just a reflection of tumor breakdown. In this context, the inhibitor activity for NEU3 was expected to be attributed to one of the low density lipoproteins,23,24 known to be major ganglioside binding proteins in vivo, but their molecular sizes were clearly distinct, although the possibility cannot be completely excluded. The presence of the inhibitor may also have some relevance to the modulation of amounts of gangliosides. In preliminary experiments, we additionally found higher sialidase activities in the serum of cancer patients, including examples with bladder, testes and renal cancers (data to be published). The values so far obtained from the ELISA as well as the sialidase activity assays did not show any statistically significant correlation with pathological stage, but more detailed examinations will be necessary to clarify this issue in the future. Further work along these lines should make an important contribution to the determination of whether the serum sialidase is a potential target for new diagnosis tests for human cancers.
Acknowledgments
We thank the technologists at the Clinical Laboratory of Miyagi Cancer Center for their great help in storage of collected serum. This research was supported in part by Grants-in-Aid for Scientific Research on FS A-Step (241FT0338) and by CREST of the Japan Science and Technology Agency.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18:750–60. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- Hakomori SI. Glycosynaptic microdomains controlling tumor cell phenotype through alteration of cell growth, adhesion, and motility. FEBS Lett. 2010;584:1901–6. doi: 10.1016/j.febslet.2009.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–96. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Takahashi K, Hata K, Shiozaki K, Yamaguchi K. Sialidase significance for cancer progression. Glycoconj J. 2012;29:567–77. doi: 10.1007/s10719-012-9394-1. [DOI] [PubMed] [Google Scholar]
- Hata K, Koseki K, Yamaguchi K, et al. Limited inhibitory effects of oseltamivir and zanamivir on human sialidases. Antimicrob Agents Chemother. 2008;52:3484–91. doi: 10.1128/AAC.00344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki K, Wada T, Hosono M, et al. Human cytosolic sialidase NEU2-low general tissue expression but involvement in PC-3 prostate cancer cell survival. Biochem Biophys Res Commun. 2012;428:142–9. doi: 10.1016/j.bbrc.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Bilyy R, Tomin A, Mahorivska I, et al. Antibody-mediated sialidase activity in blood serum of patients with multiple myeloma. J Mol Recognit. 2011;24:576–84. doi: 10.1002/jmr.1071. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Wada T, Yamaguchi K, Hata K, Shiozaki K. Plasma membrane-associated sialidase as a crucial regulator of transmembrane signaling. J Biochem. 2008;144:279–85. doi: 10.1093/jb/mvn089. [DOI] [PubMed] [Google Scholar]
- Kakugawa Y, Wada T, Yamaguchi K, et al. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc Natl Acad Sci USA. 2002;99:10718–23. doi: 10.1073/pnas.152597199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Saito S, Wada T, et al. Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility. J Biol Chem. 2006;281:7756–64. doi: 10.1074/jbc.M509668200. [DOI] [PubMed] [Google Scholar]
- Nomura H, Tamada Y, Miyagi T, et al. Expression of NEU3 (plasma membrane-associated sialidase) in clear cell adenocarcinoma of the ovary: its relationship with T factor of pTNM classification. Oncol Res. 2006;16:289–97. doi: 10.3727/000000006783981035. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Sato I, Wada T, et al. Plasma membrane-associated sialidase (NEU3) regulates progression of prostate cancer to androgen-independent growth through modulation of androgen receptor signaling. Cell Death Differ. 2012;19:170–9. doi: 10.1038/cdd.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Shiozaki K, Yamaguchi K, et al. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28:1218–29. doi: 10.1038/onc.2008.471. [DOI] [PubMed] [Google Scholar]
- Yamanami H, Shiozaki K, Wada T, et al. Down-regulation of sialidase NEU4 may contribute to invasive properties of human colon cancers. Cancer Sci. 2007;98:299–307. doi: 10.1111/j.1349-7006.2007.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. Determination of sialic acids in human serum by reversed-phase liquid chromatography with fluorimatric detection. J Chromatogr. 1992;579:209–13. doi: 10.1016/0378-4347(92)80384-3. [DOI] [PubMed] [Google Scholar]
- Galjart NJ, Gillemans N, Harris A, et al. Expression of cDNA encoding the human “protective protein” associated with lysosomal β-galactosidase and neuraminidase: homology to yeast proteases. Cell. 1988;54:755–64. doi: 10.1016/s0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Konno K, Emori Y, et al. Molecular cloning and expression of cDNA encoding rat skeletal muscle cytosolic sialidase. J Biol Chem. 1993;268:26435–40. [PubMed] [Google Scholar]
- Wang Y, Yamaguchi K, Wada T, et al. A close association of the ganglioside-specific sialidase Neu3 with caveolin in membrane microdomains. J Biol Chem. 2002;277:26252–9. doi: 10.1074/jbc.M110515200. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Wada T, Iwamatsu A, et al. Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J Biol Chem. 1999;274:5004–11. doi: 10.1074/jbc.274.8.5004. [DOI] [PubMed] [Google Scholar]
- Wada T, Yoshikawa Y, Tokuyama S, Kuwabara M, Akita H, Miyagi T. Cloning, expression, and chromosomal mapping of a human ganglioside sialidase. Biochem Biophys Res Commun. 1999;261:21–7. doi: 10.1006/bbrc.1999.0973. [DOI] [PubMed] [Google Scholar]
- Lumen N, Fonteyne V, De Meerleert G, et al. Population screening for prostate cancer: an overview of available studies and meta-analysis. Int J Urol. 2012;19:100–8. doi: 10.1111/j.1442-2042.2011.02912.x. [DOI] [PubMed] [Google Scholar]
- Katopodis N, Hirshaut Y, Geller NL, Stock CC. Lipid-associated sialic acid test for the detection of human cancer. Cancer Res. 1982;42:5270–5. [PubMed] [Google Scholar]
- Senn H-J, Orth M, Fitzke E, Wieland H, Gerock W. Gangliosides in normal human serum concentration, pattern and transport by lipoproteins. Eur J Biochem. 1989;181:657–62. doi: 10.1111/j.1432-1033.1989.tb14773.x. [DOI] [PubMed] [Google Scholar]
- Bergelson LD. Serum gangliosides as endogenous immunomodulators. Immunol Today. 1995;16:483–6. doi: 10.1016/0167-5699(95)80032-8. [DOI] [PubMed] [Google Scholar]


