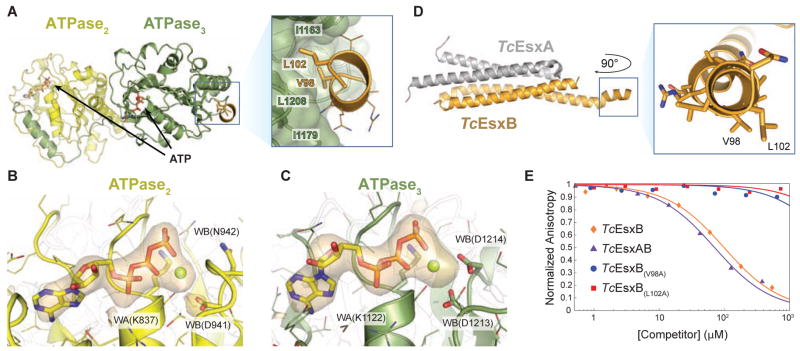
Figure 2. Co-crystal structure reveals signal sequence binding pocket in TcEccCb.
(A) The crystal structure of TcEccCb (ATPase domains colored as in Figure 1A) bound to the C-terminal signal sequence of TcEsxB (gold). Binding of the C-terminal amino acids of TcEsxB to ATPase3 is mediated by interactions with two conserved hydrophobic residues that bind in a hydrophobic binding pocket. Only the C-terminal signal sequence residues are interpretable in the electron density (Figure S2A), and the Y-X-X-X-D/E motif implicated in secretion (Daleke et al., 2012) appears disordered in the crystal. (B–C) The orange volume represents the simulated-annealing difference-density map calculated for ATPase2 ( B) and ATPase3 ( C) without nucleotide and contoured at 4 σ. (D) X-ray structure of Tc the EsxAB heterodimer with a close-up view of the C-terminal signal sequence helix. V98 and L102, which are necessary for binding to TcEccC, are labeled. (E) Binding of a fluorescently labeled signal sequence peptide (5-FAM-VNRVQALLNG) to TcEccC(cyto) monitored in the presence of increasing concentrations of unlabeled competing full length TcEsxB. Wild-type TcEsxB and TcEsxAB heterodimer compete with the peptide. Mutations in L102 or V98 prevent competition with the wild-type peptide, indicating that they do not bind. Presented data are representative experiments. Also see Figure S2.