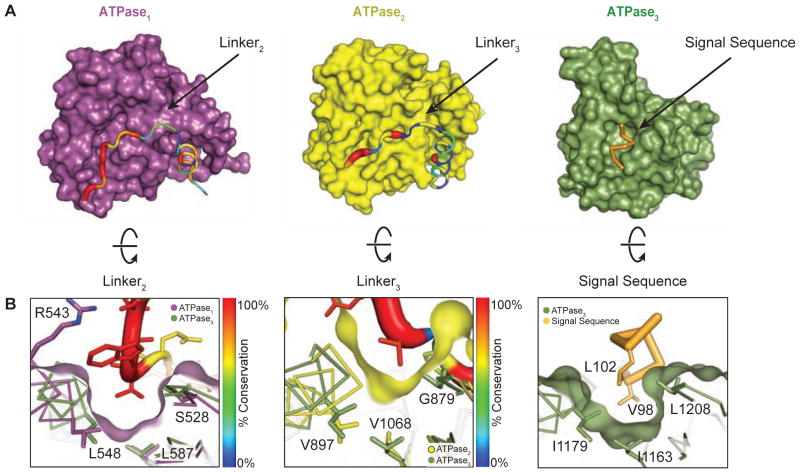
Figure 4. Residues in linker2 and linker3 mimic the substrate and bind to pocket1 and pocket2 on TcEccC.
(A) The individual ATPase domains are shown and have been rotated to reveal the path of the linker across the ATPase domain. The linker is colored and weighted in diameter according to the degree of conservation across 142 unique EccC sequences. (B) The surface has been rotated to highlight the linker groove. ATPase3 and the pocket residues (Figure S2C) overlay ATPase1 and ATPase2 to highlight the homologies in the linker binding and signal sequence binding pockets. The ATPase2 pocket is significantly shallower than ATPase1 and ATPase3.