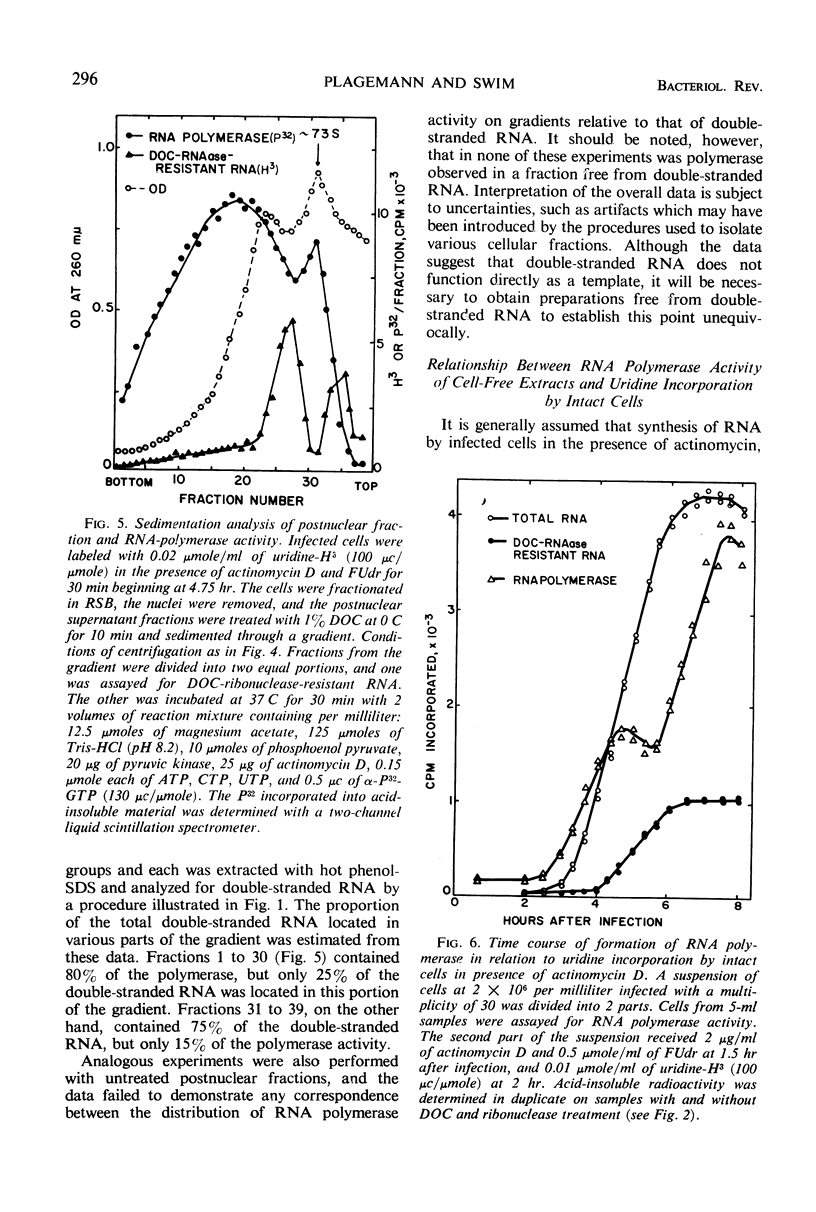
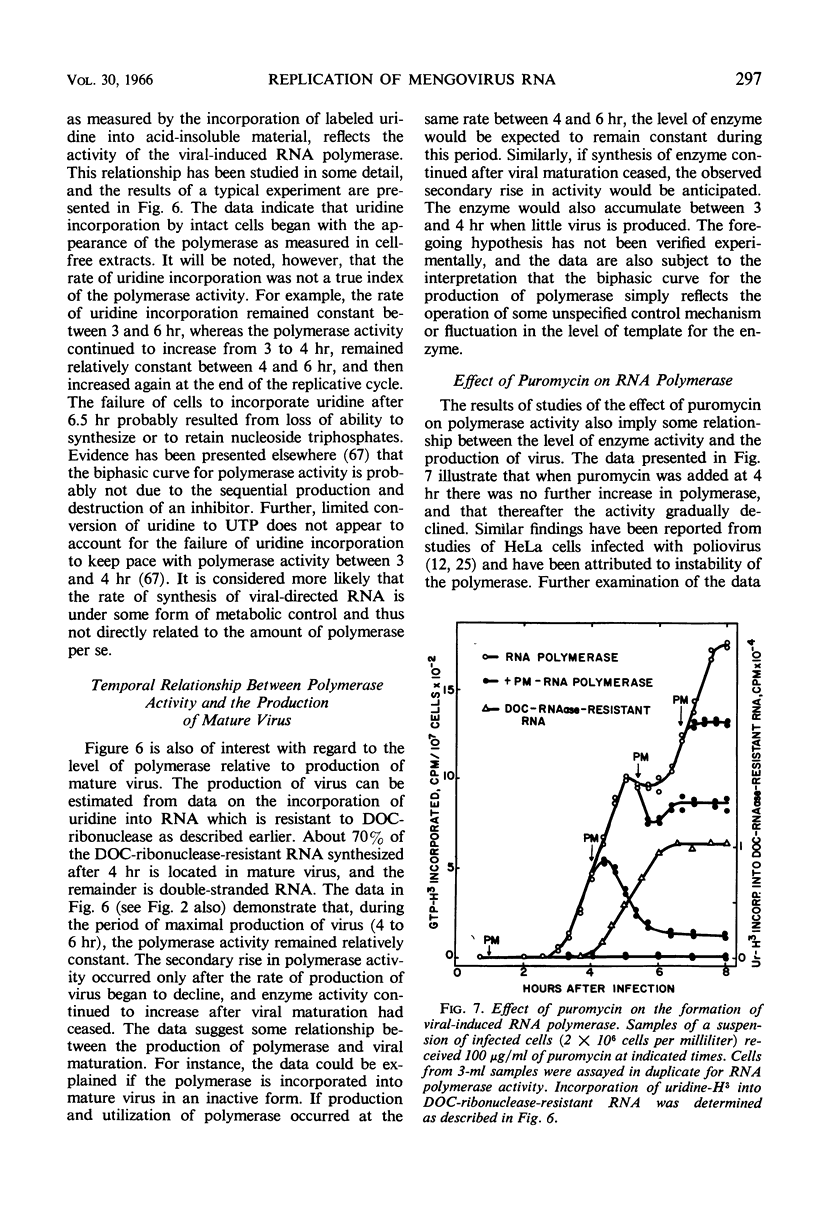
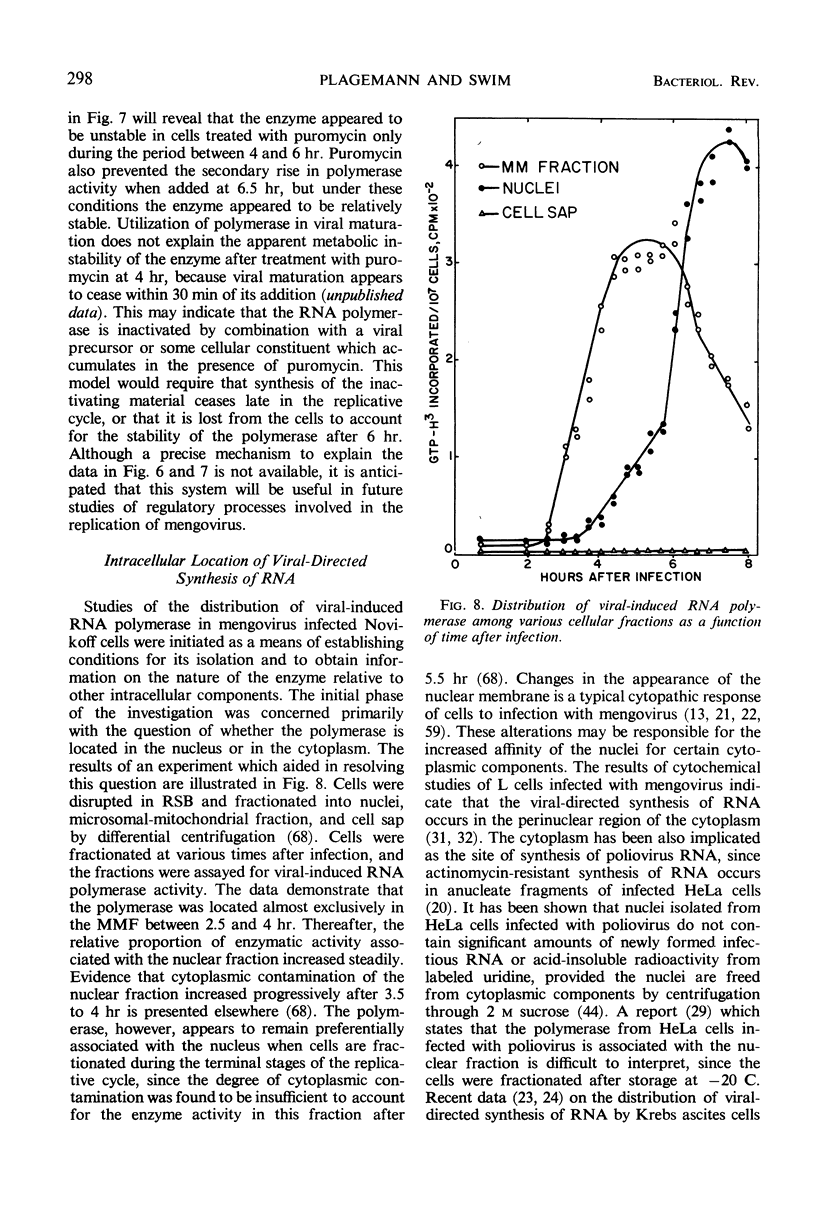
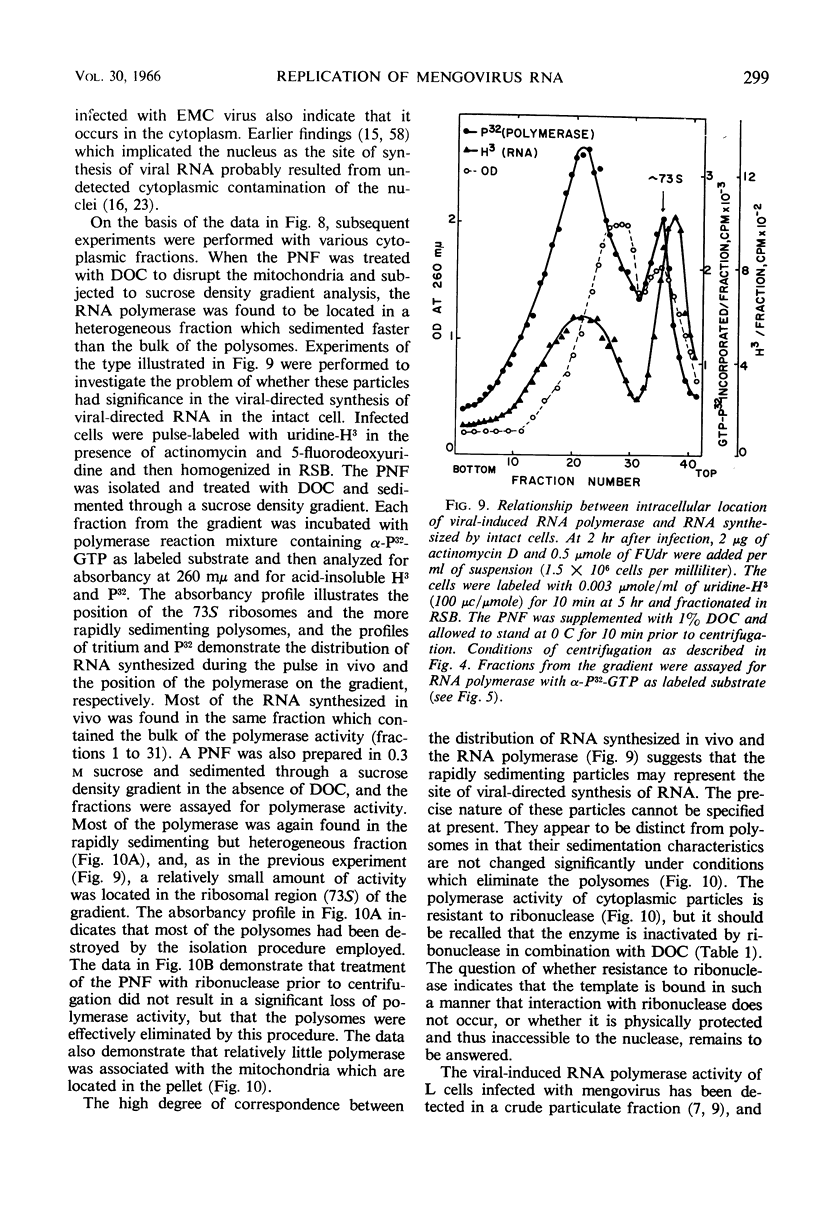
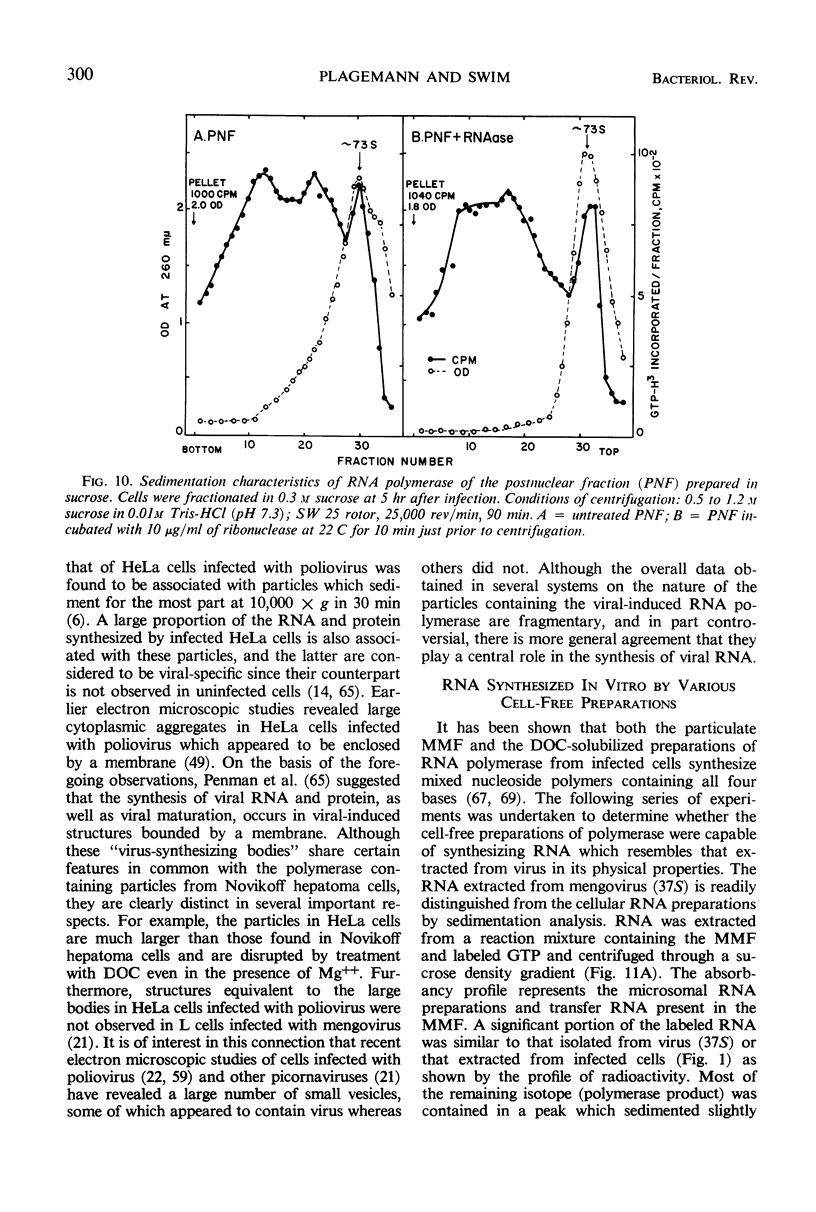
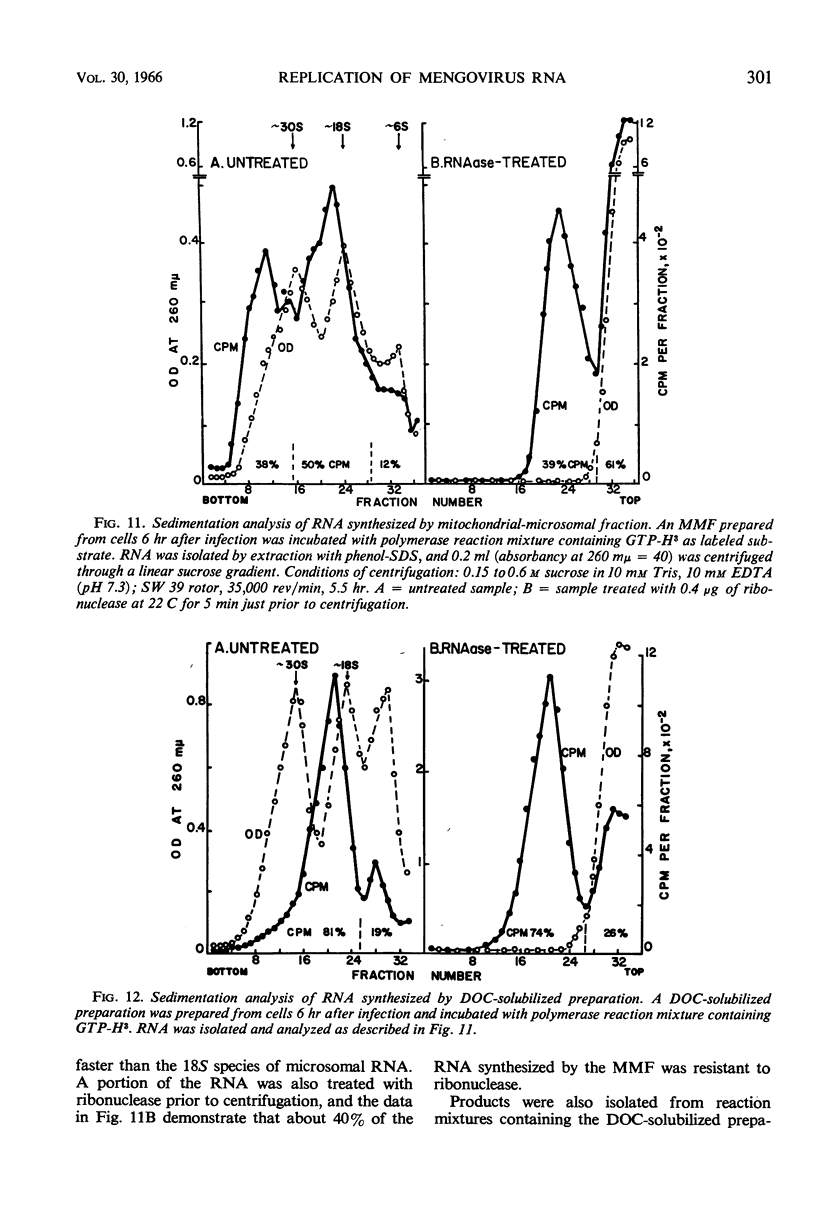
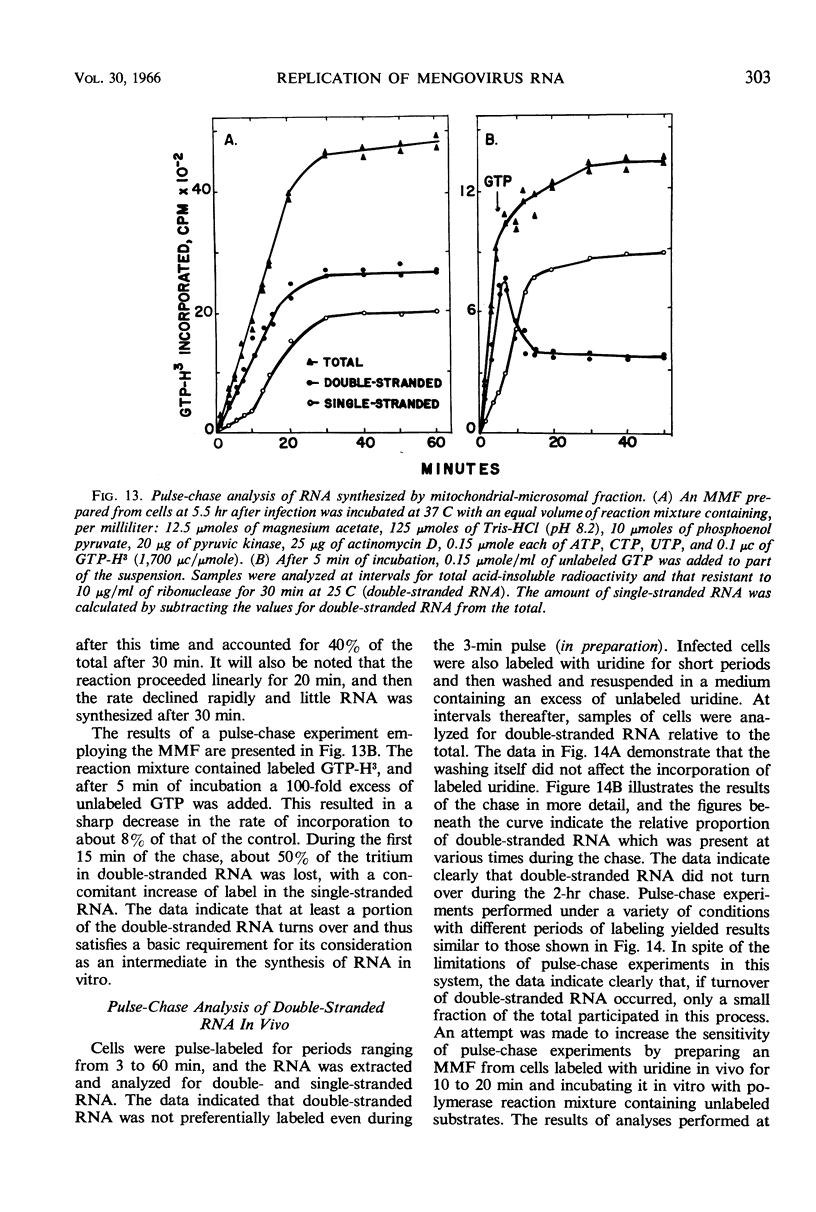
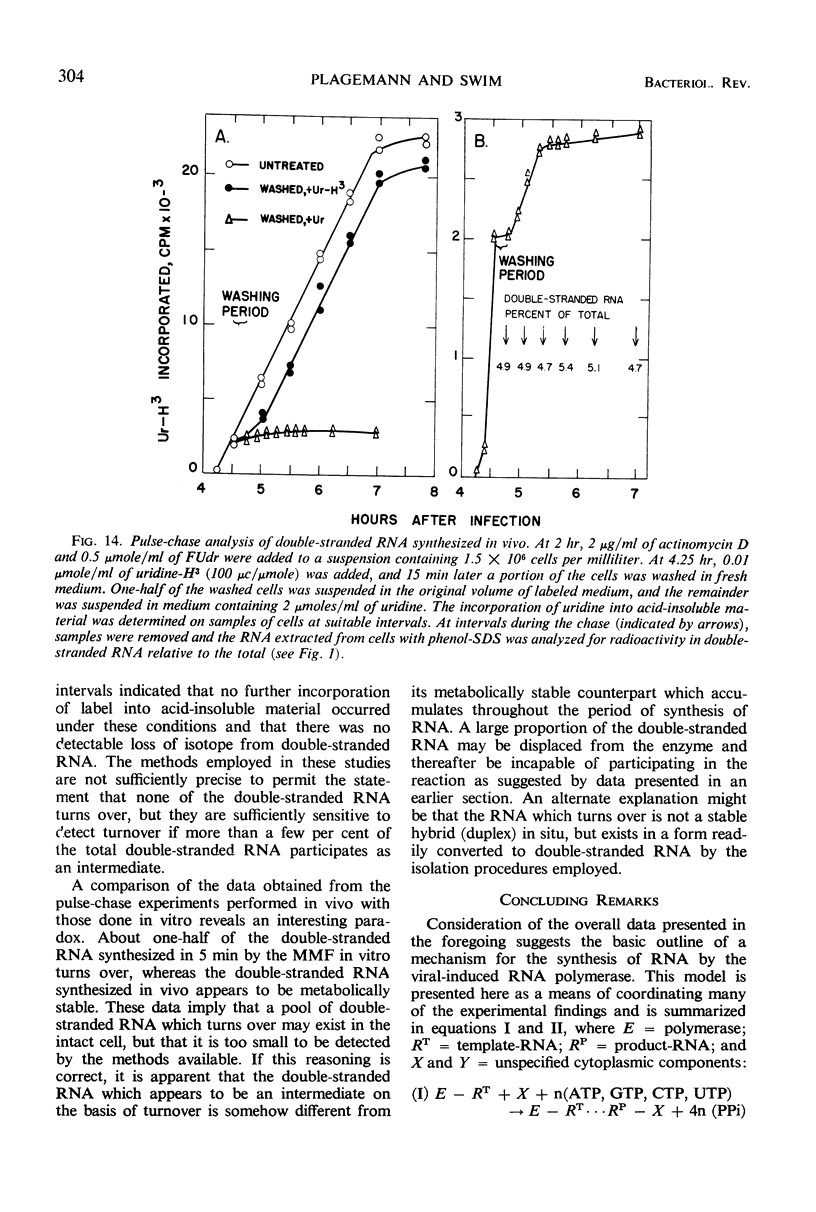
Full text
PDF

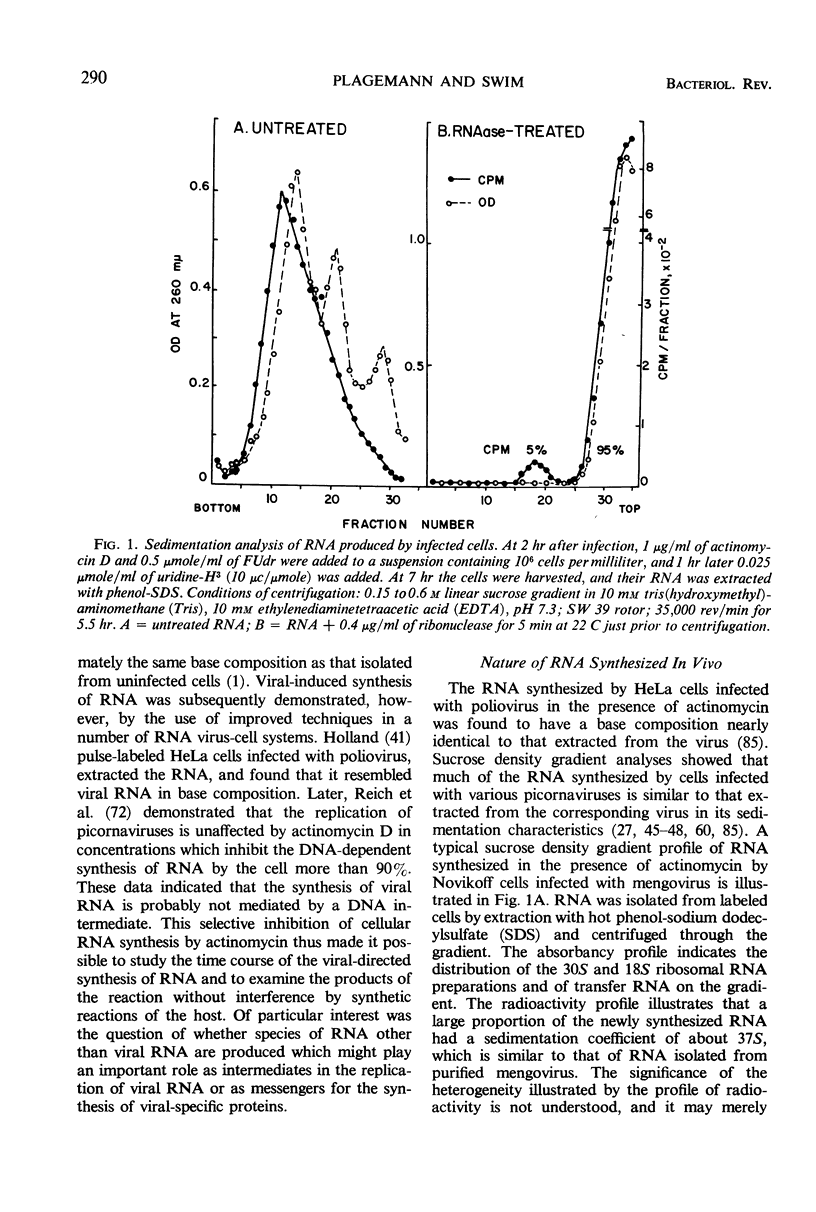
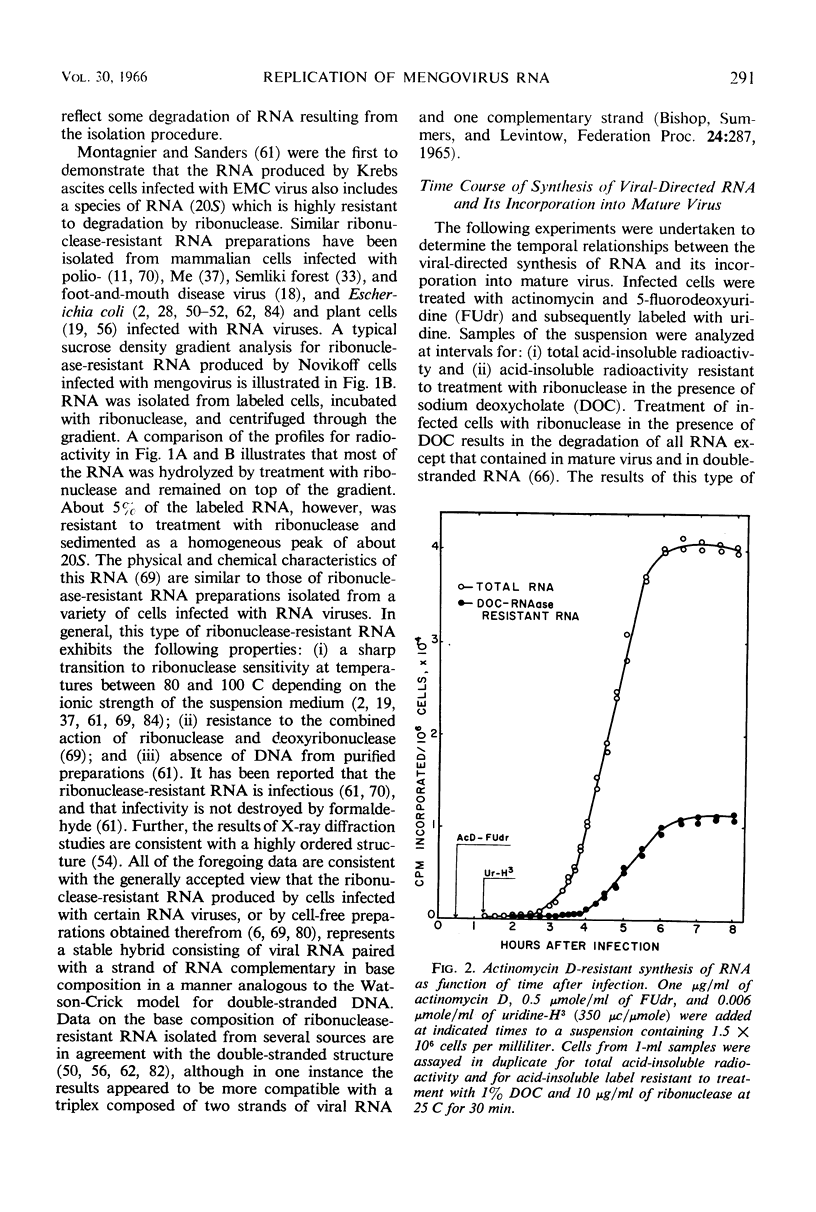
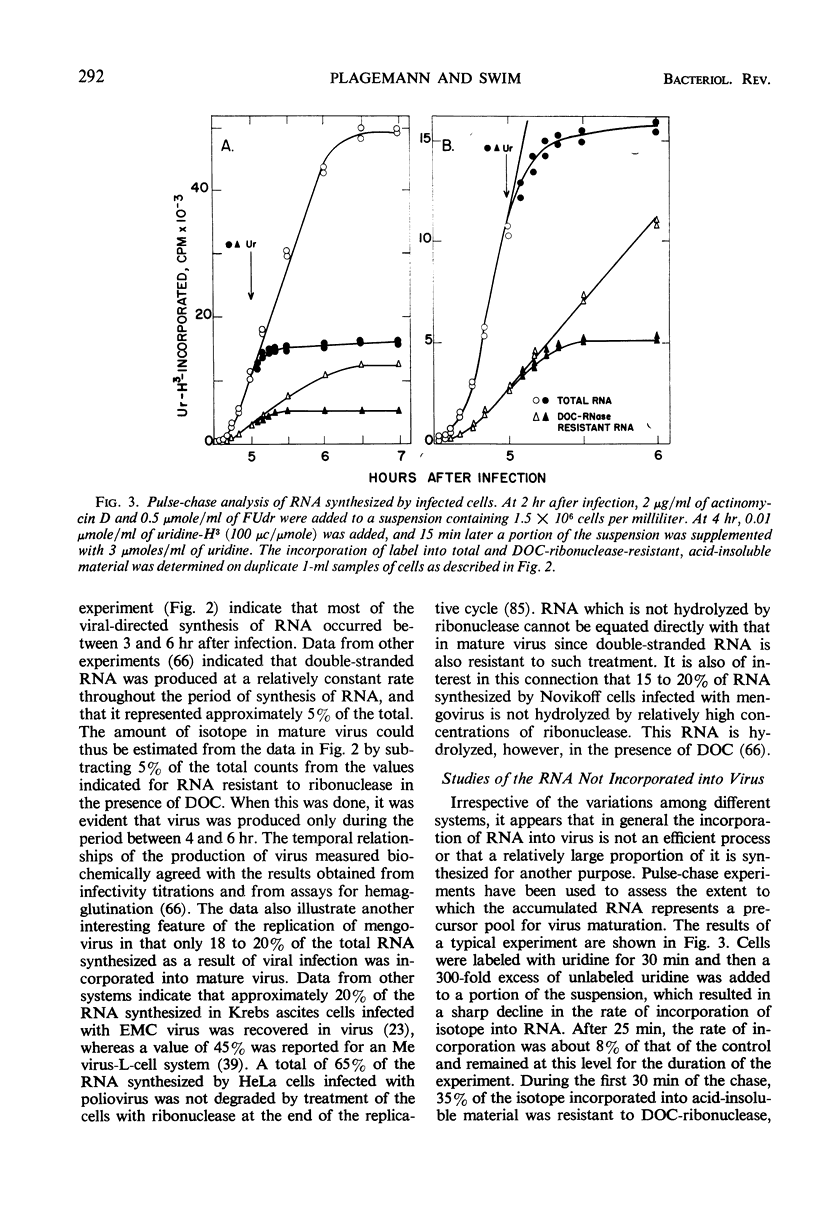
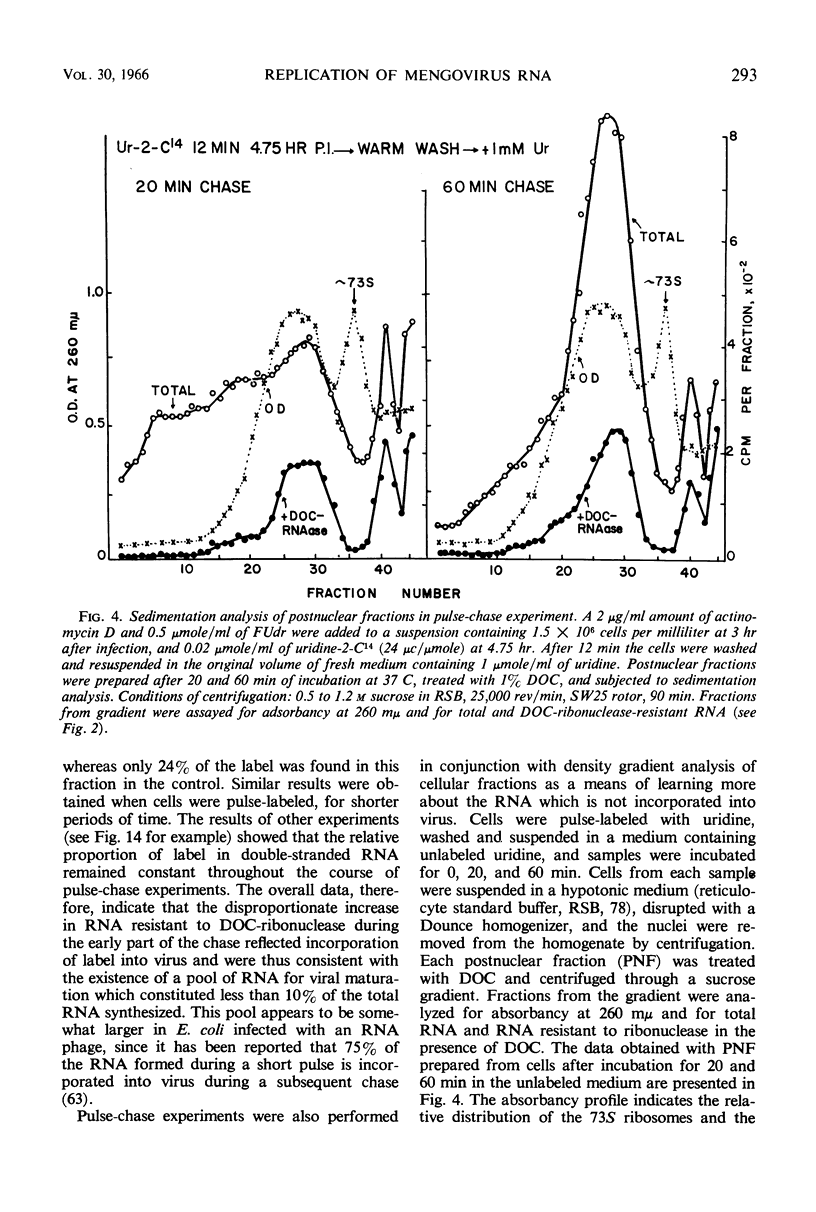







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., LOH P. C., PAYNE F. E. Studies of the biosynthesis of protein and ribonucleic acid in HeLa cells infected with poliovirus. Virology. 1959 Feb;7(2):170–183. doi: 10.1016/0042-6822(59)90185-0. [DOI] [PubMed] [Google Scholar]
- AMMANN J., DELIUS H., HOFSCHNEIDER P. H. ISOLATION AND PROPERTIES OF AN INTACT PHAGE-SPECIFIC REPLICATIVE FORM OF RNA PHAGE M12. J Mol Biol. 1964 Dec;10:557–561. doi: 10.1016/s0022-2836(64)80079-6. [DOI] [PubMed] [Google Scholar]
- ATTARDI G., SMITH J. Virus specific protein and a ribo-nucleic acid associated with ribosomes in poliovirus infected HeLa cells. Cold Spring Harb Symp Quant Biol. 1962;27:271–292. doi: 10.1101/sqb.1962.027.001.026. [DOI] [PubMed] [Google Scholar]
- AUGUST J. T., SHAPIRO L., EOYANG L. REPLICATION OF RNA VIRUSES I. CHARACTERIZATION OF A VIRAL RNA-DEPENDENT RNA POLYMERASE. J Mol Biol. 1965 Feb;11:257–271. doi: 10.1016/s0022-2836(65)80056-0. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D., BECKER Y., DARNELL J. E. VIRUS-SPECIFIC DOUBLE-STRANDED RNA IN POLIOVIRUS-INFECTED CELLS. Science. 1964 Mar 6;143(3610):1034–1036. doi: 10.1126/science.143.3610.1034. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D., EGGERS H. J., FRANKLIN R. M., TAMM I. Poliovirus-induced RNA polymerase and the effects of virus-specific inhibitors on its production. Proc Natl Acad Sci U S A. 1963 Jun;49:843–849. doi: 10.1073/pnas.49.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M. A NEW RIBONUCLEIC ACID POLYMERASE APPEARING AFTER MENGOVIRUS INFECTION OF L-CELLS. J Biol Chem. 1963 Oct;238:3395–3400. [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M., CALLENDER J. MENGOVIRUS-INDUCED INHIBITION OF HOST RIBONUCLEIC ACID AND PROTEIN SYNTHESIS. Biochim Biophys Acta. 1963 Nov 22;76:425–430. [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M. Preliminary data on a virus-specific enzyme system responsible for the synthesis of viral RNA. Biochem Biophys Res Commun. 1962 Nov 27;9:388–392. doi: 10.1016/0006-291x(62)90021-9. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M. The effect of Mengovirus infection on the activity of the DNA-dependent RNA polymerase of L-cells. Proc Natl Acad Sci U S A. 1962 Aug;48:1383–1390. doi: 10.1073/pnas.48.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTIMORE D. IN VITRO SYNTHESIS OF VIRAL RNA BY THE POLIOVIRUS RNA POLYMERASE. Proc Natl Acad Sci U S A. 1964 Mar;51:450–456. doi: 10.1073/pnas.51.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARSKI G., KINHIDA T. Continuous phase contrast observation of cells infected with Mengo-EMC viruses. Virology. 1961 Jun;14:299–301. doi: 10.1016/0042-6822(61)90211-2. [DOI] [PubMed] [Google Scholar]
- BECKER Y., PENMAN S., DARNELL J. E. A CYTOPLASMIC PARTICULATE INVOLVED IN POLIOVIRUS SYNTHESIS. Virology. 1963 Oct;21:274–276. doi: 10.1016/0042-6822(63)90271-x. [DOI] [PubMed] [Google Scholar]
- BELLETT A. J., BURNESS A. T. Intracellular sites of synthesis of encephalomyocarditis virus components in Krebs-2 ascites tumour cells. J Gen Microbiol. 1963 Jan;30:131–140. doi: 10.1099/00221287-30-1-131. [DOI] [PubMed] [Google Scholar]
- BELLETT A. J., HARRIS R. G., SANDERS F. K. SITES OF SYNTHESIS OF ENCEPHALOMYOCARDITIS VIRUS COMPONENTS IN INFECTED L-CELLS. J Gen Microbiol. 1965 Mar;38:299–307. doi: 10.1099/00221287-38-3-299. [DOI] [PubMed] [Google Scholar]
- BROWN F., CARTWRIGHT B. VIRUS SPECIFIC RIBONUCLEIC ACIDS IN BABY HAMSTER KIDNEY CELLS INFECTED WITH FOOT-AND-MOUTH DISEASE VIRUS. Nature. 1964 Nov 28;204:855–856. doi: 10.1038/204855a0. [DOI] [PubMed] [Google Scholar]
- BURDON R. H., BILLETER M. A., WEISSMANN C., WARNER R. C., OCHOA S., KNIGHT C. A. REPLICATION OF VIRAL RNA, V. PRESENCE OF A VIRUS-SPECIFIC DOUBLE-STRANDED RNA IN LEAVES INFECTED WITH TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1964 Sep;52:768–775. doi: 10.1073/pnas.52.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandin I. G., Franklin R. M. The effect of mengovirus infection on the activity of the DNA-dependent RNA polymerase of L-cells. II. Preliminary data on the inhibitory factor. Biochem Biophys Res Commun. 1964 Feb 18;15(1):27–32. doi: 10.1016/0006-291x(64)90097-x. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Kaesberg P. Isolation and properties of RNA from bromegrass mosaic virus. J Mol Biol. 1965 Aug;13(1):127–137. doi: 10.1016/s0022-2836(65)80084-5. [DOI] [PubMed] [Google Scholar]
- CROCKER T. T., PFENDT E., SPENDLOVE R. POLIOVIRUS: GROWTH IN NON-NUCLEATE CYTOPLASM. Science. 1964 Jul 24;145(3630):401–403. doi: 10.1126/science.145.3630.401. [DOI] [PubMed] [Google Scholar]
- DALES S., FRANKLIN R. M. A comparison of the changes in fine structure of L cells during single cycles of viral multiplication, following their infection with the viruses of Mengo and encephalomyocarditis. J Cell Biol. 1962 Aug;14:281–302. doi: 10.1083/jcb.14.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALGARNO L., MARTIN E. M. STUDIES ON EMC VIRAL RNA SYNTHESIS AND ITS LOCALIZATION IN INFECTED KREBS ASCITES CELLS. Virology. 1965 Jul;26:450–465. doi: 10.1016/0042-6822(65)90008-5. [DOI] [PubMed] [Google Scholar]
- EASON R., SMELLIE R. M. THE INTRACELLULAR SITE OF FORMATION OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. J Biol Chem. 1965 Jun;240:2580–2586. [PubMed] [Google Scholar]
- EGGERS H. J., BALTIMORE D., TAMM I. THE RELATION OF PROTEIN SYNTHESIS TO FORMATION OF POLIOVIRUS RNA POLYMERASE. Virology. 1963 Oct;21:281–283. doi: 10.1016/0042-6822(63)90274-5. [DOI] [PubMed] [Google Scholar]
- Enger M. D., Stubbs E. A., Mitra S., Kaesberg P. BIOPHYSICAL CHARACTERISTICS OF THE RNA-CONTAINING BACTERIAL VIRUS R17. Proc Natl Acad Sci U S A. 1963 Jun;49(6):857–860. doi: 10.1073/pnas.49.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENWICK M. L., ERIKSON R. L., FRANKLIN R. M. REPLICATION OF THE RNA OF BACTERIOPHAGE R17. Science. 1964 Oct 23;146(3643):527–530. doi: 10.1126/science.146.3643.527. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L. The influence of poliovirus infection of RNA synthesis in mammalian cells. Virology. 1963 Mar;19:241–249. doi: 10.1016/0042-6822(63)90061-8. [DOI] [PubMed] [Google Scholar]
- FLIKKE M., CHRISTIANSEN E., LAHELLE O. INTRACELLULAR DISTRIBUTION OF POLIOVIRUS INDUCED RNA-DEPENDENT RNA POLYMERASE (REPLICASE) IN DETROIT-6 CELLS DURING THE REPLICATION CYCLE. Biochem Biophys Res Commun. 1965 Jan 18;18:288–294. doi: 10.1016/0006-291x(65)90755-2. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M. A cytochemical description of the multiplication of mengovirus in L-929 cells. J Cell Biol. 1962 Jan;12:1–15. doi: 10.1083/jcb.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., ROSNER J. Localization of ribonucleic acid synthesis in mengovirus-infected L-cells. Biochim Biophys Acta. 1962 Jan 22;55:240–241. doi: 10.1016/0006-3002(62)90960-5. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition by interferon of production of double-stranded Semliki forest virus ribonucleic acid. Nature. 1965 May 1;206(983):532–532. doi: 10.1038/206532a0. [DOI] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- HARUNA I., NOZU K., OHTAKA Y., SPIEGELMAN S. AN RNA "REPLICASE" INDUCED BY AND SELECTIVE FOR A VIRAL RNA: ISOLATION AND PROPERTIES. Proc Natl Acad Sci U S A. 1963 Nov;50:905–911. doi: 10.1073/pnas.50.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSEN P., SCHAEFER W. [Studies on a mouse encephalomyelitis virus. Purification and physical-chemical properties of the virus]. Z Naturforsch B. 1962 Jan;17B:15–22. [PubMed] [Google Scholar]
- HAUSEN P. STUDIES ON THE OCCURRENCE AND FUNCTION OF VIRUS-INDUCED DOUBLE-STRANDED RNA IN THE ME-VIRUS L-CELL SYSTEM. Virology. 1965 Apr;25:523–531. doi: 10.1016/0042-6822(65)90080-2. [DOI] [PubMed] [Google Scholar]
- HAUSEN P., VERWOERD D. W. STUDIES ON THE MULTIPLICATION OF A MEMBER OF THE COLUMBIA SK GROUP (ME VIRUS) IN L CELLS. III. ALTERATION OF RNA AND PROTEIN SYNTHETIC PATTERNS IN VIRUS-INFECTED CELLS. Virology. 1963 Dec;21:617–627. doi: 10.1016/0042-6822(63)90235-6. [DOI] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H., MARVIN D. A., DUERWALD H. EIN FAEDIGER DNS-PHAGE (FD) UND EIN SPHAERISCHER RNS-PHAGE (FR), WIRTSSPEZIFISCH FUER MAENNLICHE STAEMME VON E. COLI. 1. PRAEPARATION UND CHEMISCHE EIGENSCHAFTEN VON FD UND FR. Z Naturforsch B. 1963 Nov;18:876–883. [PubMed] [Google Scholar]
- HOLLAND J. J. Altered base ratios in HeLa cell RNA during poliovirus infection. Biochem Biophys Res Commun. 1961 Nov 20;6:196–200. doi: 10.1016/0006-291x(61)90128-0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., BASSETT D. W. EVIDENCE FOR CYTOPLASMIC REPLICATION OF POLIOVIRUS RIBONUCLEIC ACID. Virology. 1964 Jun;23:164–172. doi: 10.1016/0042-6822(64)90279-x. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H., McLAREN L. C., SYVERTON J. T. Enteroviral ribonucleic acid. I. Recovery from virus and assimilation by cells. J Exp Med. 1960 Nov 1;112:821–839. doi: 10.1084/jem.112.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Inhibition of DNA-primed RNA synthesis during poliovirus infection of human cells. Biochem Biophys Res Commun. 1962 Dec 19;9:556–562. doi: 10.1016/0006-291x(62)90125-0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., PETERSON J. A. NUCLEIC ACID AND PROTEIN SYNTHESIS DURING POLIOVIRUS INFECTION OF HUMAN CELLS. J Mol Biol. 1964 Apr;8:556–575. doi: 10.1016/s0022-2836(64)80011-5. [DOI] [PubMed] [Google Scholar]
- HOMMA M., GRAHAM A. F. SYNTHESIS OF RNA IN L CELLS INFECTED WITH MENGO VIRUS. J Cell Physiol. 1963 Oct;62:179–192. doi: 10.1002/jcp.1030620207. [DOI] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Specific template requirments of RNA replicases. Proc Natl Acad Sci U S A. 1965 Aug;54(2):579–587. doi: 10.1073/pnas.54.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAERNER H. C., HOFFMANN-BERLING H. SYNTHESIS OF DOUBLE-STRANDED RNA IN RNA-PHAGE INFECTED E. COLI CELLS. Nature. 1964 Jun 6;202:1012–1013. doi: 10.1038/2021012a0. [DOI] [PubMed] [Google Scholar]
- KELLY R. B., GOULD J. L., SINSHEIMER R. L. THE REPLICATION OF BACTERIOPHAGE MS2. IV. RNA COMPONENTS SPECIFICALLY ASSOCIATED WITH INFECTION. J Mol Biol. 1965 Mar;11:562–575. doi: 10.1016/s0022-2836(65)80011-0. [DOI] [PubMed] [Google Scholar]
- KELLY R. B., SINSHEIMER R. L. A NEW RNA COMPONENT IN MS2-INFECTED CELLS. J Mol Biol. 1964 Apr;8:602–605. doi: 10.1016/s0022-2836(64)80015-2. [DOI] [PubMed] [Google Scholar]
- KERR I. M., MARTIN E. M., HAMILTON M. G., WORK T. S. The initiation of virus protein synthesis in Krebs ascites tumor cells infected with EMC virus. Cold Spring Harb Symp Quant Biol. 1962;27:259–269. doi: 10.1101/sqb.1962.027.001.025. [DOI] [PubMed] [Google Scholar]
- LANGRIDGE R., BILLETER M. A., BORST P., BURDON R. H., WEISSMANN C. THE REPLICATIVE FORM OF MS2 RNA: AN X-RAY DIFFRACTION STUDY. Proc Natl Acad Sci U S A. 1964 Jul;52:114–119. doi: 10.1073/pnas.52.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. IV. The localization of metabolic changes within subcellular fractions of Krebs II mouse-ascites-tumour cells infected with encephalomyocarditis virus. Biochem J. 1961 Dec;81:514–520. doi: 10.1042/bj0810514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTAGNIER L., SANDERS F. K. REPLICATIVE FORM OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. Nature. 1963 Aug 17;199:664–667. doi: 10.1038/199664a0. [DOI] [PubMed] [Google Scholar]
- Mandel H. G., Matthews R. E., Matus A., Ralph R. K. Replicative form of plant viral RNA. Biochem Biophys Res Commun. 1964 Aug 11;16(6):604–609. doi: 10.1016/0006-291x(64)90200-1. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Daniel W. A. Replication of poliovirus in HeLa cells: electron microscopic observations. Virology. 1965 Aug;26(4):646–663. doi: 10.1016/0042-6822(65)90328-4. [DOI] [PubMed] [Google Scholar]
- NONOYAMA M., IKEDA Y. RIBONUCLEASE-RESISTANT RNA FOUND IN CELLS OF ESCHERICHIA COLI INFECTED WITH RNA PHAGE. J Mol Biol. 1964 Sep;9:763–771. doi: 10.1016/s0022-2836(64)80181-9. [DOI] [PubMed] [Google Scholar]
- PARANCHYCH W., ELLIS D. B. IN VIVO SYNTHESIS OF PHAGE R17 RNA IN THE PRESENCE OF CHLORAMPHENICOL. Virology. 1964 Dec;24:635–644. doi: 10.1016/0042-6822(64)90218-1. [DOI] [PubMed] [Google Scholar]
- PARANCHYCH W., GRAHAM A. F. Isolation and properties of an RNA-containing bacteriophage. J Cell Comp Physiol. 1962 Dec;60:199–208. doi: 10.1002/jcp.1030600303. [DOI] [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- PONS M. INFECTIOUS DOUBLE-STRANDED POLIOVIRUS RNA. Virology. 1964 Nov;24:467–473. doi: 10.1016/0042-6822(64)90186-2. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J Bacteriol. 1966 Jun;91(6):2317–2326. doi: 10.1128/jb.91.6.2317-2326.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. II. General properties of the viral-induced ribonucleic acid polymerase. J Bacteriol. 1966 Jun;91(6):2327–2332. doi: 10.1128/jb.91.6.2327-2332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M. Effect of mitomycin C on the growth of some animal viruses. Proc Natl Acad Sci U S A. 1961 Aug;47:1212–1217. doi: 10.1073/pnas.47.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M., SHATKIN A. J., TATUMEL Action of actinomycin D on animal cells and viruses. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1238–1245. doi: 10.1073/pnas.48.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., LOCKART R. Z., Jr, SEBRING E. D. Alterations in HeLa cell metabolism resulting from poliovirus infection. Virology. 1959 Oct;9:244–259. doi: 10.1016/0042-6822(59)90118-7. [DOI] [PubMed] [Google Scholar]
- SCHAFFER F. L. Physical and chemical properties and infectivity of RNA from animal viruses. Cold Spring Harb Symp Quant Biol. 1962;27:89–99. doi: 10.1101/sqb.1962.027.001.012. [DOI] [PubMed] [Google Scholar]
- SCHOLTISSEK C., ROTT R., HAUSEN P., HAUSEN H., SCHAEFER W. Conparative studies of RNA and protein synthesis with a myxovirus and a small polyhedral virus. Cold Spring Harb Symp Quant Biol. 1962;27:245–257. doi: 10.1101/sqb.1962.027.001.024. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BILLETER M. A., SCHNEIDER M. C., KNIGHT C. A., OCHOA S. REPLICATION OF VIRAL RNA. VI. NUCLEOTIDE COMPOSITION OF THE REPLICATIVE FORM OF TOBACCO MOSAIC VIRUS RNA AND OF ITS COMPONENT STRANDS. Proc Natl Acad Sci U S A. 1965 Mar;53:653–656. doi: 10.1073/pnas.53.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA. IV. PROPERTIES OF RNA SYNTHETASE AND ENZYMATIC SYNTHESIS OF MS2 PHAGE RNA. Proc Natl Acad Sci U S A. 1964 May;51:890–897. doi: 10.1073/pnas.51.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P. DOUBLE-STRANDED RIBONUCLEIC ACID FORMATION IN VITRO BY MS 2 PHAGE-INDUCED RNA SYNTHETASE. Science. 1963 Nov 29;142(3596):1188–1191. doi: 10.1126/science.142.3596.1188. [DOI] [PubMed] [Google Scholar]
- WEISSMANN C., SIMON L., OCHOA S. Induction by an RNA phage of an enzyme catalyzing incorporation of ribonucleotides into ribonucleic acid. Proc Natl Acad Sci U S A. 1963 Mar 15;49:407–414. doi: 10.1073/pnas.49.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C. Replication of viral RNA, VII. Further studies on the enzymatic replication of MS2 RNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):202–207. doi: 10.1073/pnas.54.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., HEETER M., DARNELL J. E. RNA synthesis in poliovirus-infected cells. Virology. 1963 Mar;19:400–408. doi: 10.1016/0042-6822(63)90080-1. [DOI] [PubMed] [Google Scholar]


