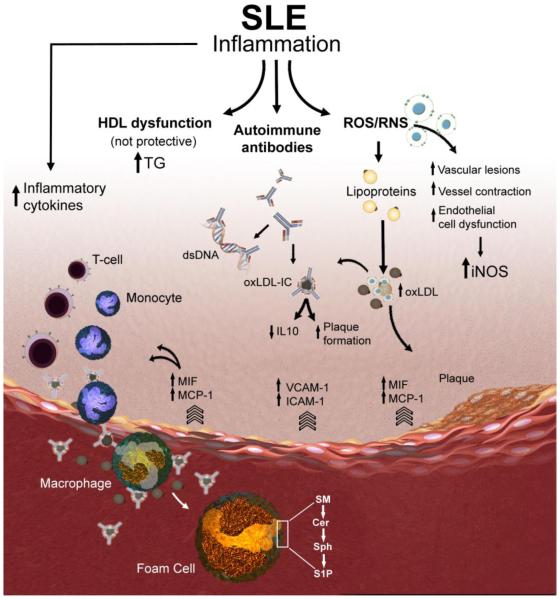
Figure 1. Schematic representation of putative macrophage-related mechanisms contributing to accelerated atherosclerosis in SLE.
Atherosclerotic plaques begin with the recruitment of inflammatory cells such as monocytes and T cells to the endothelial lining of the blood vessel. The endothelial cells release leukocyte adhesion molecules (e.g., VCAM-1, ICAM-1 and E-selectin) to facilitate sub-endothelial cell migration. MIF and MCP-1 levels are elevated in SLE patients and lead to increased pro-inflammatory cytokines. Increase in the production of reactive oxygen and nitrogen species (ROS and RNS) has been implicated in vascular lesion formation. LDL modified by the effect of ROS results in the formation of oxidized LDL (oxLDL). SLE and other autoimmune diseases produce autoantibodies which form complexes with either lipid metabolizing enzymes like lipoprotein lipase or with apolipoproteins and slow down lipoprotein catabolism, which may produce varieties of dyslipidemia. oxLDL complexed to IgG (oxLDL-IC) can be internalized by macrophages and results in the release of pro-inflammatory cytokines and promotes plaque formation. Pro-inflammatory HDL has been strongly associated with the development of carotid plaques and with intima media thickness in SLE patients. Targeting key metabolizing enzymes in the sphingolipid pathway (e.g., sphingomyelinases, ceramidases, and sphingosine kinases) that regulate the generation of the bioactive lipids sphingomyelin (SM), ceramide (Cer), sphingosine (Sph), and sphingosine 1- phosphate (S1P) in activated macrophages and foam cells might avert accelerated vascular disease in SLE.