Abstract
Tracking and predicting motor outcomes is important in determining effective stroke rehabilitation strategies. Diffusion tensor imaging (DTI) allows for evaluation of the underlying structural integrity of brain white matter tracts and may serve as a potential biomarker for tracking and predicting motor recovery. In this study, we examined the longitudinal relationship between DTI measures of the posterior limb of the internal capsule (PLIC) and upper-limb motor outcomes in 13 stroke patients (median 20-month post-stroke) who completed up to 15 sessions of intervention using brain–computer interface (BCI) technology. Patients’ upper-limb motor outcomes and PLIC DTI measures including fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) were assessed longitudinally at four time points: pre-, mid-, immediately post- and 1-month-post intervention. DTI measures and ratios of each DTI measure comparing the ipsilesional and contralesional PLIC were correlated with patients’ motor outcomes to examine the relationship between structural integrity of the PLIC and patients’ motor recovery. We found that lower diffusivity and higher FA values of the ipsilesional PLIC were significantly correlated with better upper-limb motor function. Baseline DTI ratios were significantly correlated with motor outcomes measured immediately post and 1-month-post BCI interventions. A few patients achieved improvements in motor recovery meeting the minimum clinically important difference (MCID). These findings suggest that upper-limb motor recovery in stroke patients receiving BCI interventions relates to the microstructural status of the PLIC. Lower diffusivity and higher FA measures of the ipsilesional PLIC contribute toward better motor recovery in the stroke-affected upper-limb. DTI-derived measures may be a clinically useful biomarker in tracking and predicting motor recovery in stroke patients receiving BCI interventions.
Keywords: DTI, fractional anisotropy, axial diffusivity, radial diffusivity, mean diffusivity, motor recovery, stroke rehabilitation, brain-computer interface
Introduction
One of the most common deficits following stroke is upper-limb motor impairment, which can have a significant impact on disability and health (Coupar et al., 2012). Rehabilitation is often scheduled for patients to minimize disability and to improve the quality of patients’ daily living activities. Estimating patient’s potential for motor function recovery is critical in making decisions on the type, duration and goals of rehabilitation (Stinear, 2010). Previous studies have reported that stroke location rather than the volume of an infarct was more important for predicting functional recovery (Pineiro et al., 2000; Crafton et al., 2003). Furthermore, the posterior limb of the internal capsule (PLIC) was significantly associated with poor recovery of isolated upper-limb movements (Shelton and Reding, 2001). Given the significance of PLIC involved in motor impairment and recovery, one aim of this study is to systematically assess the structural integrity of the PLIC using diffusion tensor imaging (DTI). DTI is a non-invasive brain imaging technique that allows for quantitative evaluation of the structural integrity of white matter tracts after a stroke (Werring et al., 2000; Stinear et al., 2007; Yu et al., 2009). A recent study shows evidence that DTI measures may be used as potential biomarkers for predicting stroke recovery in stroke patients receiving transcranial direct current stimulation (Lindenberg et al., 2012). BCI facilitated intervention is a novel neurorehabilitation therapy that has drawn increasing attention in recent years. BCI devices provide real-time feedback to assist users to learn modulation of brain activity. This type of modulation and further enhancement with motor training may promote brain plasticity changes and eventually boost the recovery when patients have reached a functional plateau with more traditional therapies (Fallani et al., 2013; Varkuti et al., 2013). To date, few studies have examined the white matter structural integrity in stroke patients receiving BCI intervention.
We have recently proposed that FA is a valuable measure for examining the microstructural integrity of the PLIC and a promising biomarker in tracking and predicting motor functional recovery in stroke patients receiving BCI interventions (Song et al., 2014). In this study, we systematically and longitudinally examined the structural integrity of the PLIC in 13 stroke patients who completed up to 15 sessions of BCI intervention facilitated by functional electrical muscle stimulation (FES). This analysis included measures of fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) and ratio of each of these measures between the ipsilesional and contralesional PLIC. We further evaluated the predictive value of these DTI measures on upper-limb motor impairment and functional recovery in stroke patients receiving BCI interventions to determine whether they are clinically meaningful predictors of motor recovery.
Materials and Methods
Patients
Thirteen patients with stroke were included into this study. This study was approved by the University of Wisconsin-Madison’s Institutional Review Board. All patients provided written informed consent. Standard clinical MRI was utilized to assess damage to PLIC by a neuroradiologist (VP). Ten of the 13 patients showed damage to the PLIC due to stroke. Patients CI003 with a minor left frontal lobe infarct, CI010 with right occipital stroke and CT003 with a right pontine infarct did not show damage to the PLIC. Patient CT004 with a left middle cerebral artery (MCA) territory infarct showed minimal damage to the PLIC. A summary of patient characteristics is given in Table 1.
Table 1.
Patient characteristics.
| Patient ID | Age/years | Gender | Months since stroke | Baseline NIHSS | Baseline NIHSS-motor arm | Lesion location |
|---|---|---|---|---|---|---|
| CI001 | 52 | M | 15 | 8 | 4 | Left MCA |
| CI002 | 62 | F | 16 | 8 | 4 | Left precentral gyrus |
| CI003 | 68 | M | 3 | 0 | 0 | Left frontal lobe |
| CI004 | 66 | M | 23 | 6 | 1 | Left MCA |
| CI005 | 73 | F | 2 | 0 | 0 | Left MCA |
| CI007 | 59 | M | 28 | 2 | 0 | Left temporal lobe |
| CI008 | 45 | F | 99 | 6 | 2 | Right frontoparietal infarct |
| CI009 | 71 | F | 26 | 6 | 2 | Right temporal-frontal-parietal lobe |
| CI010 | 80 | M | 20 | 2 | 0 | Right occipital lobe |
| CT001 | 75 | F | 23 | 7 | 3 | Right putamen |
| CT002 | 55 | M | 17 | 0 | 0 | Left basal ganglia |
| CT003 | 49 | M | 6 | 3 | 1 | Right pons |
| CT010 | 50 | M | 26 | 4 | 3 | Right MCA |
| Mean ± SD | 61.9 ± 11.21 | 5 F/8 M | 23.4 ± 24.33 | 4.0 ± 3.03 | 1.5 ± 1.56 | 7 L/ 6 R |
MCA, middle cerebral artery.
Study Design
Diffusion tensor imaging data and motor outcome assessments were acquired at four time points: before the start of intervention (i.e., immediately pre-intervention), at the midpoint of intervention (i.e., mid-intervention), upon completion of intervention phase (i.e., immediately post-intervention), and 1 month following the last session of BCI intervention (i.e., 1-month-post-intervention). A detailed description of the study design and the procedure of BCI intervention is elaborated in references (Song et al., 2014; Young et al., 2014a,b). Each patient was administered up to fifteen 2-h sessions of interventional BCI therapy (14.08 ± 1.71 sessions). These sessions took place over a period of up to 6 weeks with two to three intervention sessions per week.
Patient inclusion criteria include: (1) ages 18 years and above; (2) no known neurologic, psychiatric or developmental disability; (3) persistent upper-extremity motor impairment resulting from ischemic or hemorrhagic stroke. Exclusion criteria include: (1) contraindications for MRI; (2) allergy to electrode gel, surgical tape and metals that would be used in BCI intervention; (3) under treatment for infectious disease or having apparent oral lesions or inflammation.
Motor Outcome Measures
Each patient was assessed for clinical stroke severity in addition to neurological examination at four time points throughout the BCI intervention. The overall neurological deficit and severity of motor paresis was evaluated using the National Institute of Health Stroke Scale (NIHSS; Brott et al., 1989). Upper-limb functional performance was assessed by the Action Research Arm Test (ARAT). The ARAT is a standardized performance measure of upper-limb function assessing grasp, grip, pinch and gross arm movement. The ARAT scores range from 0 to 57. Besides an objective evaluation of motor outcomes using ARAT, we included a subjective measure of the Stroke Impact Scale (SIS) hand function subscale (SIS-hand function). SIS-hand function was used to assess self-reported satisfaction with hand use. Raw scores of SIS-hand function were transformed (Stewart and Ware, 1992) with a transformed scale = 100 × [(actual raw score – lowest possible raw score)/possible raw score]. The transformed SIS-hand function scores range from 0 to 100. All clinical assessments of the stroke-affected limb for each patient at each time point are given in Table 2.
Table 2.
Clinical motor outcome assessments of the stroke-affected limb.
| Subject ID | Time points | ARAT | SIS Hand function |
|---|---|---|---|
| CI001 | Pre-therapy | 0 | 0 |
| Mid-therapy | 3 | 0 | |
| Immediately post-therapy | 0 | 0 | |
| One-month-post-therapy | 0 | 0 | |
| CI002 | Pre-therapy | 0 | 0 |
| Mid-therapy | 0 | 0 | |
| Immediately post-therapy | 0 | 0 | |
| One-month-post-therapy | 0 | 0 | |
| CI003 | Pre-therapy | 57 | 40 |
| Mid-therapy | 57 | 55 | |
| Immediately post-therapy | 57 | 70 | |
| One-month-post-therapy | 57 | 75 | |
| CI004 | Pre-therapy | 3 | 0 |
| Mid-therapy | 0 | 0 | |
| Immediately post-therapy | 0 | 0 | |
| One-month-post-therapy | 0 | 0 | |
| CI005 | Pre-therapy | 56 | 50 |
| Mid-therapy | 46 | 70 | |
| Immediately post-therapy | 54 | 50 | |
| One-month-post-therapy | 57 | 57.5 | |
| CI007 | Pre-therapy | 57 | 55 |
| Mid-therapy | 57 | 100 | |
| Immediately post-therapy | 57 | 70 | |
| One-month-post-therapy | 57 | 65 | |
| CI008 | Pre-therapy | 3 | 0 |
| Mid-therapy | 5 | 0 | |
| Immediately post-therapy | 4 | 0 | |
| One-month-post-therapy | 5 | 0 | |
| CI009 | Pre-therapy | 3 | 0 |
| Mid-therapy | 3 | 10 | |
| Immediately post-therapy | 0 | 5 | |
| One-month-post-therapy | 3 | 10 | |
| CI010 | Pre-therapy | 57 | 55 |
| Mid-therapy | 54 | 60 | |
| Immediately post-therapy | 57 | 70 | |
| One-month-post-therapy | 57 | 70 | |
| CT001 | Pre-therapy | 0 | 5 |
| Mid-therapy | 0 | 0 | |
| Immediately post-therapy | 0 | 0 | |
| one-month-post-therapy | 0 | 0 | |
| CT002 | Mid-therapy | 53 | 75 |
| Immediately post-therapy | 57 | 75 | |
| One-month-post-therapy | 54 | 75 | |
| CT003 | Pre-therapy | 27 | 10 |
| Mid-therapy | 28 | 30 | |
| Immediately post-therapy | 40 | 35 | |
| One-month-post-therapy | 43 | 45 | |
| CT010 | Pre-therapy | 3 | 20 |
| Mid-therapy | 5 | 30 | |
| Immediately post-therapy | 4 | 15 | |
| One-month-post-therapy | 8 | 30 |
DTI Data Acquisition and Processing
DTI data was acquired on a 3 T whole-body MRI scanner (GE DISCOVERY MR750, General Electric Medical Systems, Waukesha, WI, USA) with an 8-channel head coil. MR diffusion imaging parameters were: single-shot echo planar imaging (EPI), TR = 9000 ms, TE = 66.2 ms, single average (NEX = 1), field of view (FOV) = 256 × 256 mm2, voxel size = 1 × 1 × 2 mm3, 75 axial slices with no gap between slices, flip angle = 90°, 56 gradient encoded directions, b value = 1000 s/mm2.
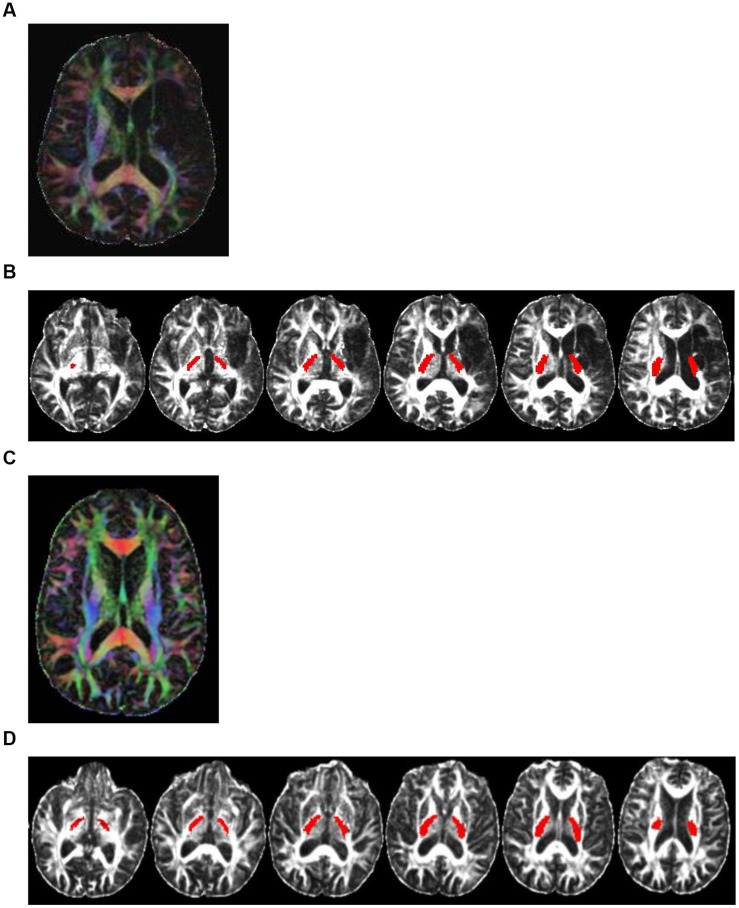
DTI data was processed using FSL (v5.0; Smith et al., 2004) with the following steps: (1) correction for motion and eddy current distortion; (2) head-motion correction for gradient direction vectors; (3) image registration and brain mask extraction; (4) estimation of tensor diffusion and generating diffusivity measures; (5) registration of standard white-matter atlases (JHU-ICBM-FA-2mm.nii.gz and JHU-ICBM-labels-2mm.nii.gz) to each patient’s native space; step 5 provided automatically segmented white-matter tracts with corresponding labels for identifying the regions of interest such as PLIC (Figure 1); (6) estimation of diffusivity for each hemispheric PLIC; (7) calculation of ratios of DTI measures (rFA, rAD, rRD, rMD) between the ipsilesional and contralesional PLIC as rValue = Valueipsi/ Valuecontra (Kusano et al., 2009; Koyama et al., 2012).
FIGURE 1.
Color-coded diffusion tensor imaging (DTI) images and the PLIC white-matter tract. Directionally encoded color (DEC) map of DTI images and the PLIC white-matter tract. (A) A DTI image of a representative patient (CI001) with damage to PLIC. Color codes to give diffusion tensor directions: red represents tracts running left to right; green is anterior to posterior; blue is superior to inferior. (B) Axial view of the PLIC white-matter tract (in red) of patient CI001. (C) A DEC map of DTI image of a representative patient (CI003) with no damage to PLIC. Color also represents diffusion tensor directions. (D) Axial view of the PLIC white-matter tract (in red) of patient CI003.
Statistical Analyses
Statistical analysis was performed using RStudio (version 0.98.1062). The threshold for statistical significance was set to p-value ≤ 0.05. A one-sided Wilcoxon signed-rank test was used to compare each DTI measure between the ipsilesional and contralesional PLIC. A Kruskal–Wallis test which is a non-parametric equivalent of ANOVA was used to evaluate the time effect on the motor outcome performances during the course of intervention. Spearman’s rank correlation coefficients were computed to examine correlations between diffusivity and motor outcome measures. To take advantage of a longitudinal, repeated-measurement design of this study, we used a generalized estimating equation (GEE) for regression analysis (Ballinger, 2004). GEEs take into account the dependency of repeated measurements from the same patient in the regression analysis and thus allow for evaluation of the relationship between DTI and motor outcome measures longitudinally. A linear regression analysis was also computed to investigate whether baseline DTI measures predicted post-intervention motor outcomes.
Results
Patient Motor Outcome Measures
Motor outcome measures are summarized in Table 2. The ARAT scores varied from zero, indicating no ability to perform the task, to maximum of 57, indicating unimpaired performance. The SIS-hand function measure varied widely, with a value of zero indicating a patient reporting no ability to use the impaired hand, and higher positive values indicating decreasing levels of difficulty using the impaired hand in daily activities such as carrying heavy objects, turning a doorknob, opening a can or jar, tying a shoe lace and picking up a dime. Although group performance on the ARAT and SIS-hand function measures was not significantly different over the course of BCI intervention (Kruskal–Wallis test, p-value > 0.05), 7 of 13 patients reported improved hand function based on their SIS-hand function scores measured at 1-month-post intervention. Three of these seven patients (CI005, CI007, and CT003) also achieved improvements meeting the minimum clinically important difference (MCID) of SIS-hand function (cutoff value = 17.8; Lin et al., 2010) at mid-intervention. Similarly, 5 of 13 patients had improved ARAT measures at 1-month-post intervention compared to baseline measures. One patient (CT003) achieved improvements meeting the MCID of ARAT (cutoff value = 5.7) at 1-month-post intervention.
Increased Diffusivity and Decreased FA Measures in Ipsilesional PLIC
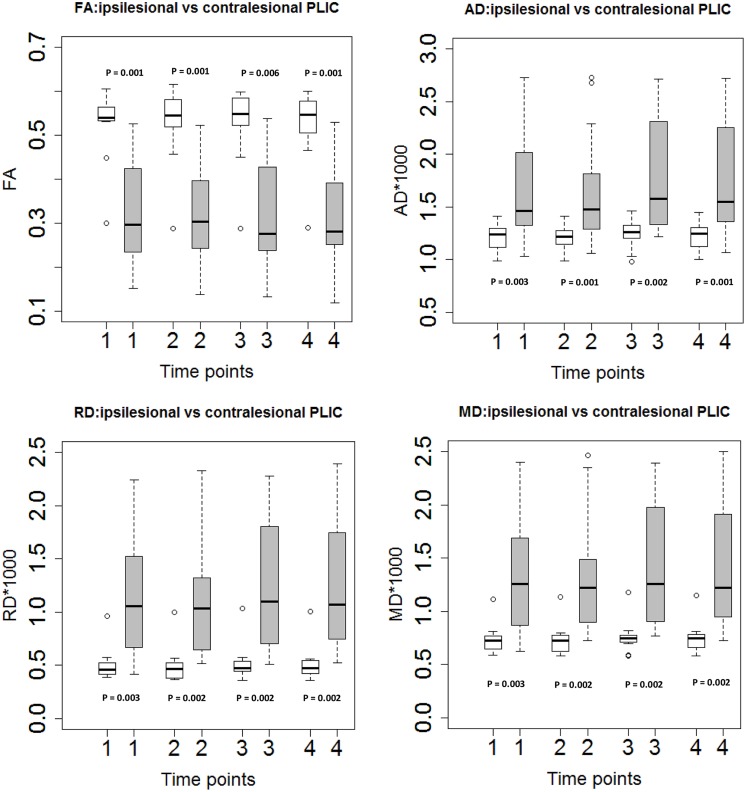
The mean values of AD, RD, and MD were higher on the ipsilesional PLIC than on the contralesional side, while FA was lower in the ipsilesional PLIC than in the controlesional PLIC (Wilcoxon signed-rank test, p-values < 0.05; Figure 2).
FIGURE 2.
Diffusion tensor imaging measures in ipsilesional and contralesional PLIC. Boxplots showed significantly higher FA, lower diffusivity measures (AD, RD, and MD) in the ipsilesional PLIC (Wilcoxon signed-rank tests).Time of immediately pre-, mid-, immediately post- and 1-month-post intervention are indicated as time-point 1, 2, 3, and 4, respectively. White boxes represent the contralesional side and gray boxes represent the ipsilesional side of PLIC.
Correlation of DTI Measures with Motor Functional Recovery
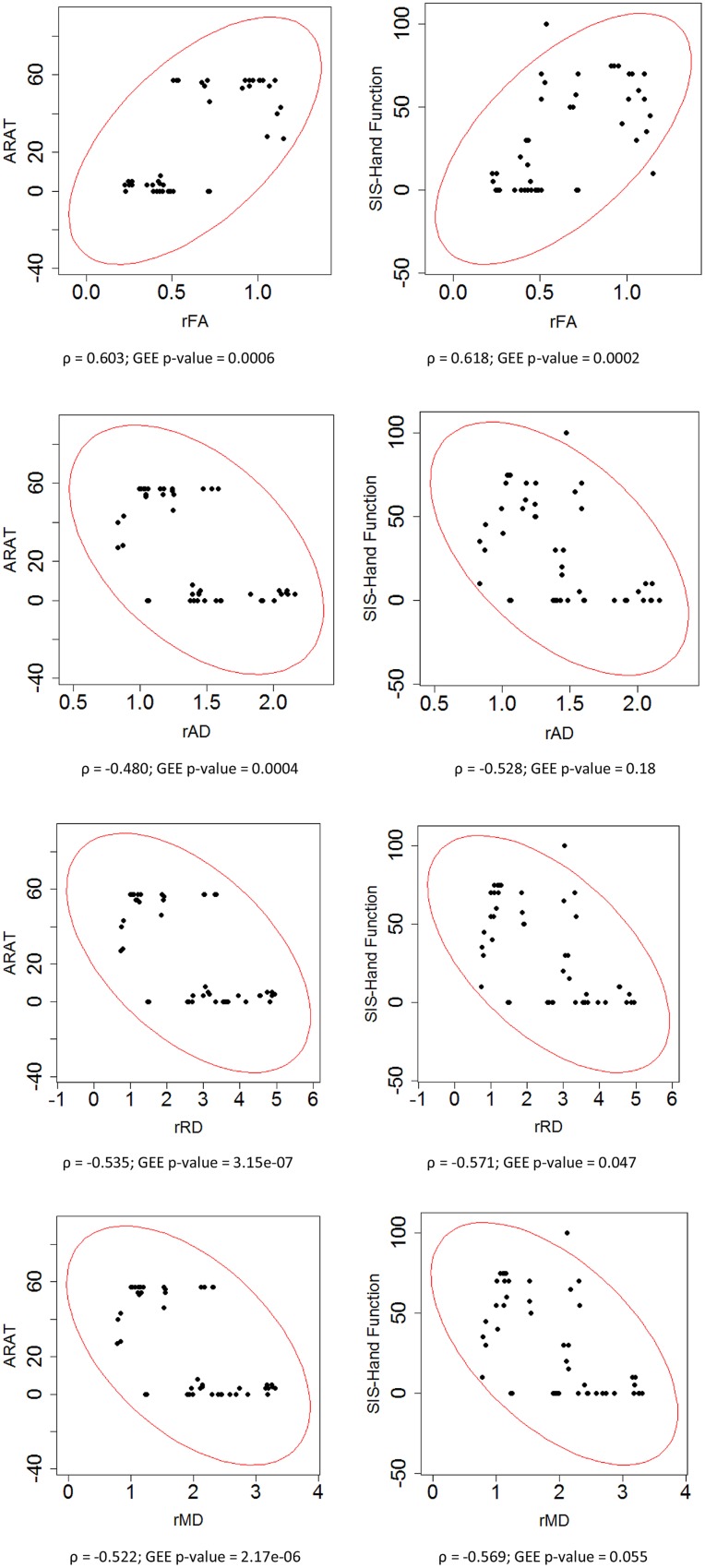
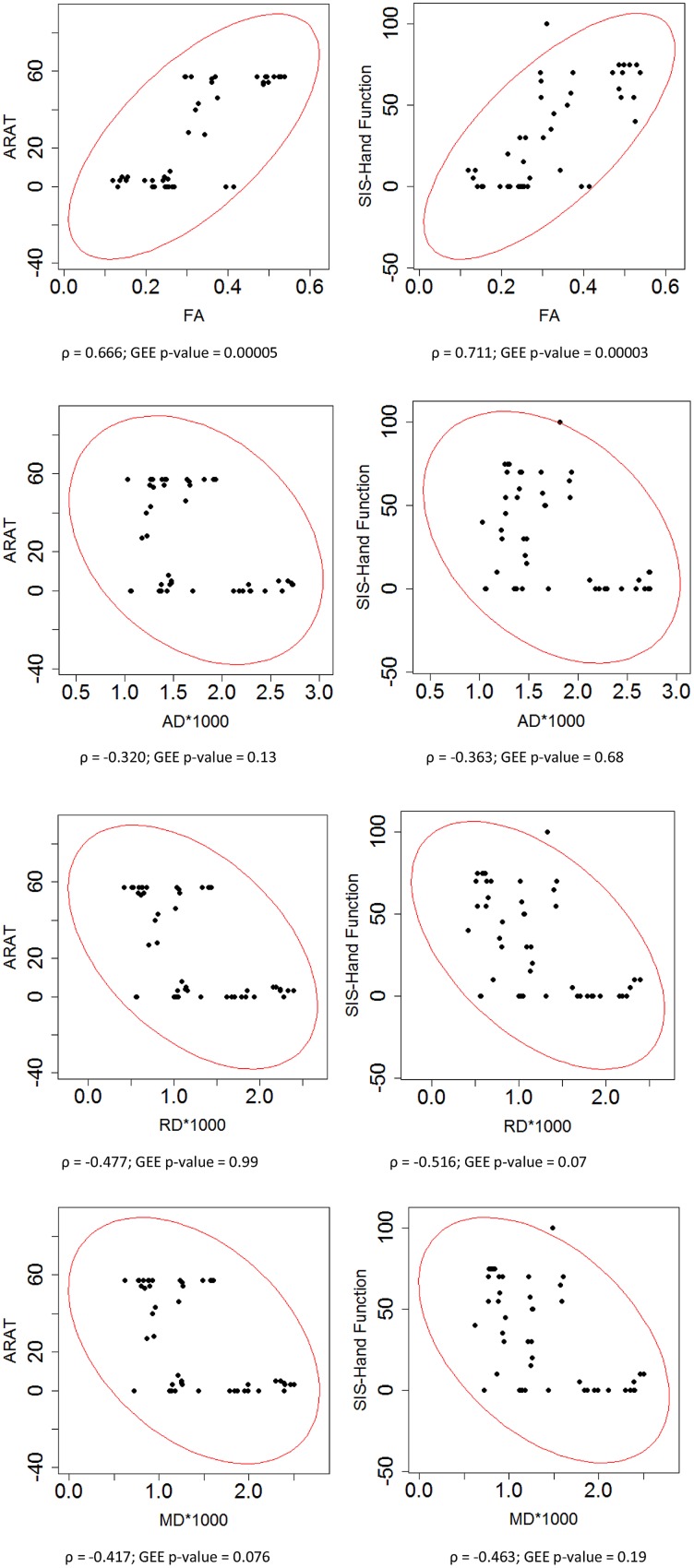
To assess the relationship between PLIC DTI and motor outcome measures, Spearman’s rank correlation coefficients were first computed for all time points of the longitudinal data acquired from all patients. This correlation analysis showed that higher ARAT scores and higher SIS-hand function scores, indicating better performance, were significantly correlated with lower diffusivity ratios (i.e., rAD, rRD, and rMD) but higher FA ratio (i.e., rFA) in the ipsilesional PLIC (Figure 3). A secondary statistical analysis, the GEE analysis, was further computed to account for the dependence of repeated measurements from each patient at different time points. These analyses confirmed that the relationships observed between DTI and objective motor outcome measures (i.e., ARAT) remained statistically significant (Table 3). We performed the same analyses to estimate the correlations between baseline DTI measures and motor outcomes. Only baseline PLIC FA was shown to be significantly correlated with motor outcome measures (Figure 4).
FIGURE 3.
Correlation of ratios of each DTI measure and motor outcomes across time. Each ratio of DTI measures was correlated with motor outcome measures including ARAT and SIS hand function scores assessed in impaired hand. Spearman rank correlation coefficient (ρ) and the statistical significance of correlation (GEE p-value) are presented.
Table 3.
Correlation coefficients of DTI measures and motor outcomes.
| Motor outcome measure | Correlation analysis | rFA | rAD | rRD | rMD |
|---|---|---|---|---|---|
| ARAT | Spearman’s rank correlation coefficients | 0.603 | -0.480 | -0.535 | -0.522 |
| GEE p-value | 0.0006 | 0.0004 | 3.15e-07 | 2.17e-06 | |
| SIS-hand function | Spearman’s rank correlation coefficients | 0.618 | -0.528 | -0.571 | -0.569 |
| GEE p-value | 0.0002 | 0.18 | 0.047 | 0.055 |
Bold values indicate significant GEE tests with p-value less than 0.05.
FIGURE 4.
Correlation of DTI measures and motor outcomes across time. Each DTI measure was correlated with motor outcome measures including ARAT and SIS hand function scores assessed in impaired hand. Spearman rank correlation coefficient (ρ) and the statistical significance of correlation (GEE p-value) are presented.
Prediction of Motor Functional Recovery Using Baseline DTI Measures
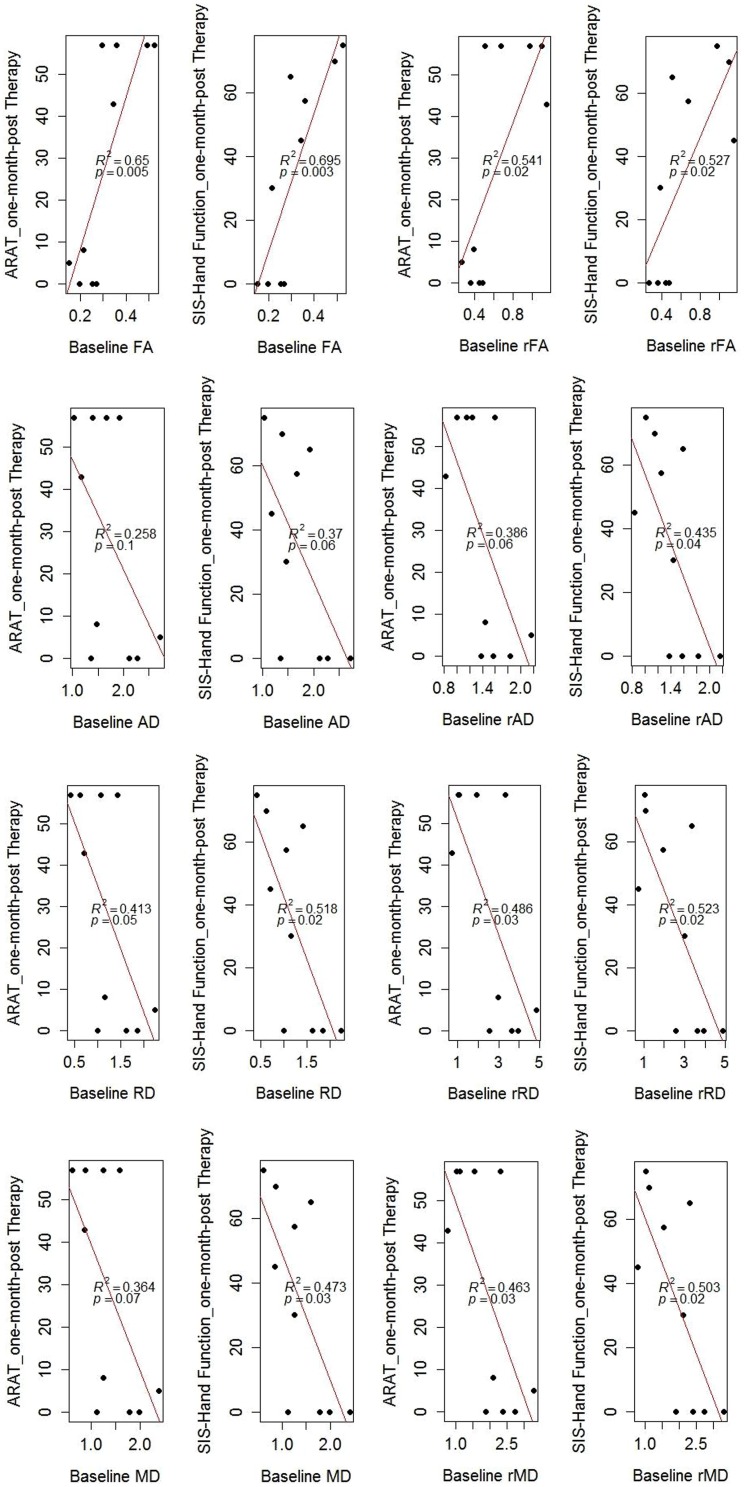
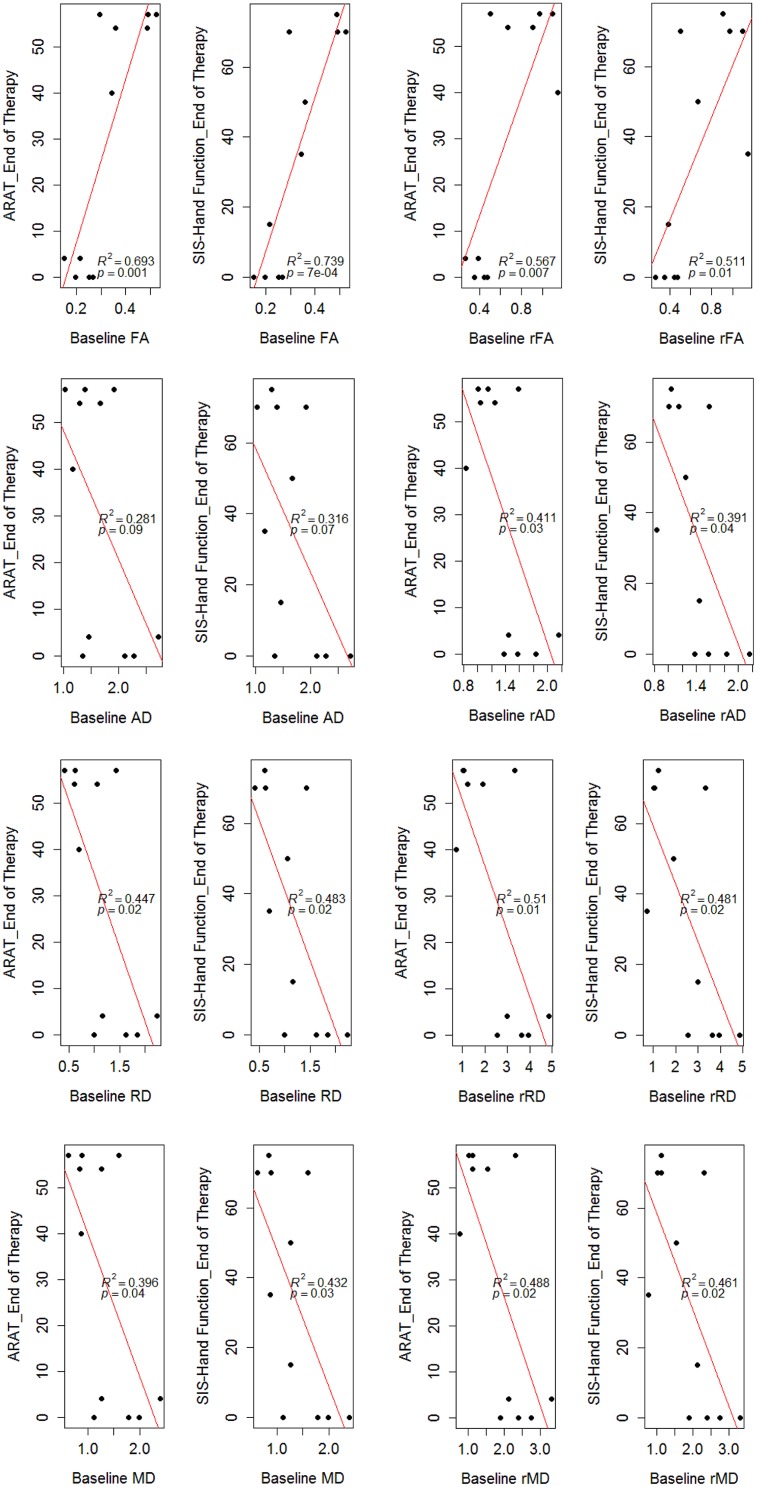
In the regression model, baseline DTI measures of the ipsilesional PLIC correlated with ARAT and SIS-hand function scores tested 1-month-post intervention (Figure 5). Diffusivity ratios (i.e., rAD, rRD, rMD) outperformed diffusivity measures (i.e., AD, RD, MD) in the regression model (Figure 5). FA remained the best predictor of motor recovery among these DTI measures (r-squared > 0.6 and p-value < 0.05). In addition, we examined whether baseline DTI measures of the ipsilesional PLIC were correlated with motor outcomes evaluated immediately post-intervention and observed very similar findings (Figure 6).
FIGURE 5.
Correlation of baseline DTI measures and motor outcomes evaluated 1-month-post intervention. Baseline DTI measures (i.e., evaluated at immediately pre-intervention) correlated with motor outcomes measured 1-month-post intervention. R-squared values (R2) and p-values of the regression model are shown on the figures. Note: there were three patients (CI002, CI009, and CT002) missing DTI scans at 1-month-post therapy, resulting 10 data points as seens in the figures.
FIGURE 6.
Correlation of baseline DTI measures and motor outcomes evaluated immediately post-intervention. Baseline DTI measures (i.e., evaluated at immediately pre-intervention) correlated with motor outcomes measured immediately post-intervention. R-squared values (R2) and p-values of the regression model are shown on the figures. There were two patients (CI002 and CI009) missing DTI scans at immediately post-therapy, resulting 11 data points as seens in the figures.
Discussion
PLIC in Motor Recovery
Injury to the corticospinal pathway from the primary motor cortex through the PLIC to the lower motor neurons can have significant impact on motor performance and functional recovery in stroke patients. In the majority of stroke patients, the upper-limb is more severely involved than the lower limb, with upper-extremity impairment more commonly seen with MCA strokes (Shelton and Reding, 2001). Studies using MRI suggest that motor impairment after stroke is clearly related to lesion size and location (Chen et al., 2000; Shelton and Reding, 2001), cortical activation at rest (Carter et al., 2003) and during voluntary movement (Ward et al., 2003), and the structural integrity of descending motor fibers (Pineiro et al., 2000; Shelton and Reding, 2001; Schiemanck et al., 2008). Previous studies also report that stroke location rather than the volume of an infarct is more important for predicting functional outcomes (Pineiro et al., 2000; Crafton et al., 2003). Shelton and Reding (2001) have observed that patients with purely cortical strokes have better motor outcome than patients with purely subcortical stroke. Patients with mixed cortical and subcortical stroke tended to do better than patients with purely subcortical stroke despite the expected larger size of mixed lesions (Shelton and Reding, 2001). Interestingly, they found that the PLIC was the only structure significantly associated with poor recovery of isolated upper-limb movements as assessed by the Fugl-Meyer motor score (Sanford et al., 1993). In another study investigating the predictive value of hemispheric stroke localization for the recovery of hand function 1 year post-stroke, Schiemanck et al. (2008) found that lesions of the internal capsule were associated with a significantly lower probability of return of isolated hand motor function than lesions of the cortical, subcortical regions and corona radiata. Although both observed significant relationships between lesion location (i.e., PLIC) and upper-limb motor recovery, these two studies did not quantify the structural integrity of the PLIC nor did they examine the relationship between the structural integrity of the PLIC and motor recovery. Given the significance of the PLIC to motor recovery, in this study, we systematically examined the structural integrity of the PLIC using DTI and investigated whether the structural integrity of the PLIC correlated with upper-limb motor outcomes and thereby affected motor recovery in stroke patients receiving BCI interventions. We found that the structural integrity of the PLIC was significantly affected by stroke (i.e., increased diffusivity and decreased FA values in the ipsilesional PLIC) and was significantly correlated with upper-limb motor outcomes. These observations show that upper-limb motor recovery could depend on the preservation of the PLIC, which provides important information for designing and evaluating post-stroke rehabilitation.
Application of DTI in Stroke
Diffusion tensor imaging is a promising MRI technique for characterizing microstructural changes in many neuropathologic conditions, and has recently been applied to evaluate the relationship between the integrity of corticospinal fiber tracts and motor outcome measures in acute and chronic stroke phases (Schaechter et al., 2009; Lindenberg et al., 2010; Koyama et al., 2012). The vast majority of DTI studies on prediction of stroke outcomes have used FA. FA evaluation is often related to white matter integrity and is considered a unique and sensitive indicator of axonal loss, while its specificity for axonal loss is not clear, because it may also be sensitive to myelination status and other abnormalities (Mori, 2006). To better understand the underlying pathology in white matter integrity, several studies have evaluated directional diffusivities such as AD, RD, and MD in both animal (Song et al., 2002, 2003) and human stroke studies (Boespflug et al., 2011; Lindenberg et al., 2012; Song et al., 2012). AD has been related to axonal damage while RD is more specific to myelination. Alteration of diffusivities in the corticospinal system due to stroke has been observed in both human and animal studies (Liu et al., 2007; Kusano et al., 2009; Schaechter et al., 2009; Lindenberg et al., 2012). In our study, DTI analyses on 13 stroke patients with varying location and size of infarct in the corticospinal system yielded consistent observations – increased diffusivities and decreased FA in the ipsilesional PLIC compared with the contralesional side (Figure 2). This phenomenon has been suggested as a characteristic of chronic white matter degeneration (Yu et al., 2009; Lindenberg et al., 2012) and is thought to arise from the loss of the tissue’s structural integrity (Liu et al., 2007).
Tracking Motor Recovery Using DTI
In additional to its role as a structural integrity indicator, DTI measures linearly correlate with motor impairment and motor function (Schaechter et al., 2009; Lindenberg et al., 2010, 2012; Boespflug et al., 2011; Koyama et al., 2012, 2013). Our study investigated this relationship in stroke patients receiving BCI interventions and found that ratios of DTI measures between ipsilesional and contralesional PLIC were significantly correlated with measures of motor function (i.e., ARAT) and self-reported hand impairment (i.e., SIS-hand function) during the course of intervention (Figure 3). More specifically, higher ARAT scores, indicating better upper-limb functioning, and higher SIS-hand function scores, indicating greater satisfaction with hand use, were significantly correlated with lower diffusivity ratio but higher FA ratio in ipsilesional PLIC. Given our observation that ipsilesional PLIC had increased diffusivity and decreased FA compared with the contralesional side, these findings suggest that the more the DTI measures of the ipsilesional PLIC resembled those observed in the contralesional PLIC, the greater the potential for functional recovery in stroke-affected limb.
We observed that ratios of diffusivity yielded stronger correlations with motor outcomes than pure diffusivity measures (Figures 3 and 4). This might be due to the fact that diffusivity ratios provided a normalized directional diffusivity measure by taking into account the underlying changes in contralesional side during stroke recovery. Furthermore, each diffusivity ratio correlated with motor outcomes which might indicate white matter remodeling in the ipsilesional and contralesional hemispheres during the rehabilitation period. Interestingly, both FA and rFA had a significant and strong correlation with motor outcomes. FA itself is a normalized scalar index proportional to the standard deviation of three eigenvalues, while pure diffusivity measures are combinations of eigenvalues and not normalized (Basser and Pierpaoli, 1996; Alexander et al., 2007).
Predicting Motor Recovery Using DTI
One of the most important findings of this study is the predictive value of DTI measures to functional motor recovery following BCI facilitated FES of the impaired arm. Baseline DTI measures, especially the ratio of each DTI measure comparing the ipsilesional and contralesional PLIC, significantly predicted motor outcomes assessed 1-month-post intervention (p-values ≤ 0.05; Figure 5). Similar findings were also observed for baseline DTI measures and motor outcomes assessed immediately post-intervention (Figure 6). This supports the potential clinical utility of DTI measures to predict upper-limb motor functional recovery of stroke patients receiving BCI and other motor recovery interventions.
Limitations
Small sample size (n = 13) and the heterogeneity of stroke patients were the primary limitations of this study. Lesion location varied in these patients. Seven of 13 patients had left hemisphere stroke and six of them had right hemisphere stroke. Within the 13 patients, three patients had subcortical stroke. A wide range of time since stroke onset was another limitation. Four patients were severely impaired and exhibited no or little improvement in functional recovery as assessed by clinical behavioral performance. These patients were minimally able to perform the designed intervention tasks, resulting in a floor effect in outcome measurements. While changes in DTI measurements were observed across time, a Kruskal–Wallis test did not show these changes to be significant. There are many factors that may result in the absence of significant changes in DTI measures across time such as the small sample size, high between-patient variance in terms of age, stroke severity and duration of stroke, and the sensitivity of the current DTI technique used in the study. Future studies will target a more homogeneous subtype of stroke patients.
There are other factors that could be useful for predicting outcomes such as age, duration of stroke, stroke severity. In the current study, we are specifically interested in the value of PLIC DTI measures in predicting motor outcomes. We performed additional analyses using age, duration of stroke (e.g., months since stroke onset) and stroke severity (e.g., baseline NIHSS-motor arm measures) as covariates, and found that ipsilesional FA, RD, and MD, age, months since stroke and motor arm scores of NIHSS baseline measures and interaction between any two of these parameters all significantly correlated with ARAT measures (p-values < 0.05).
The current study was initially designed as a crossover study with a small group of control subjects (see Supplementary Data). We compared motor outcome performances between the intervention (nine patients) and the control group (seven patients) who did not receive BCI intervention and found significant improvements only at mid-intervention. We also examined the relationship between FA and motor outcomes at individual time points at pre-, mid-, immediately post, and 1-month-post intervention. We observed changes in regression slope and correlation coefficients between FA and motor outcome measures across intervention and especially at immediately post intervention, suggesting that BCI intervention may have an effect on motor recovery compared to pre-intervention. These results can be seen in the Supplementary Data. It is worth noting that these results were preliminary with a small sample size and the analysis was done under the assumption of a linear relationship between FA and motor outcome measures. Additional data is needed for further validation.
Conclusion
In this study, we investigated the relationship between DTI and motor outcome measures in order to track and predict motor functional recovery in a group of stroke patients with persistent upper extremity impairment receiving BCI intervention facilitated with FES of the impaired arm. Given the previously established significance of PLIC involvement in motor recovery, this study evaluated stroke-induced changes in structural integrity of the PLIC using DTI measures and also investigated if these changes were related to motor recovery after a course of BCI interventions. We observed that ratio of each DTI measure was significantly correlated with motor outcomes, with lower diffusivity and higher FA measures of the ipsilesional PLIC correlating with better motor recovery. Interestingly, we also observed that baseline PLIC DTI measures assessed pre-intervention significantly correlated with motor outcomes measured immediately post and 1-month-post intervention, suggesting a predictive value of PLIC DTI in motor recovery, especially in stroke patients receiving BCI-FES interventions.
Author Contributions
JS: Assisted with patient recruitment, data collection, data analysis, and writing.
VN: Assisted with patient recruitment, data collection, and data analysis.
BY: Assisted with patient recruitment and data collection.
LW, ZN, and AR: Assisted with data collection.
MT: Provided part of system hardware and expertise.
DE: Assisted with study design and outcome measure selection and interpretation.
KC: Assisted with patient recruitment.
JAS: Assisted with study design and patient recruitment.
JW: This author is one of two lead PI’s on this project and supervised the technical and engineering aspects of the work.
VP: This author is one of two lead PI’s on this project and supervised the neuroimaging aspects of this work.
Conflict of Interest Statement
There is one patentpending on the closed-loop neurofeedback device used for the BCI-facilitated intervention administered in this study (Pending U.S. Patent Application No. 12/715,090). This patent was filed jointly by the two lead investigators Justin C. Williams and Vivek Prabhakaran. Otherwise, the authors have no conflicts of interest to report, as this research was conducted in the absence of commercial and financial relationships that might compromise the integrity of the results reported herein.
Acknowledgments
The authors thank all patients and their families for their participation. Portions of this work were previously presented in poster or e-poster form at the SFN Annual Meeting 2012, the International Stroke Conference 2013, the ISMRM Annual Meeting 2013, a platform talk at the RSNA Annual Meeting 2013 and the 2014 ASNR Annual Meeting. This work was supported by NIH grants RC1MH090912-01, K23NS086852, T32GM008692, UL1TR000427, and T32EB011434, by a Coulter Translational Research Award, an American Heart Association Postdoctoral Fellow Research Award, UW Milwaukee-Madison Intercampus Grants, by funding from the UW Graduate School, and by Grants from the Shapiro Foundation and Foundation of ASNR award.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnhum.2015.00195/abstract
References
- Alexander A. L., Lee J. E., Lazar M., Field A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics 4 316–329 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger G. A. (2004). Using generalized estimating equations for longitudinal data analysis. Organ. Res. Methods 7 127–150 10.1177/1094428104263672 [DOI] [Google Scholar]
- Basser P. J., Pierpaoli C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B 111 209–219 10.1006/jmrb.1996.0086 [DOI] [PubMed] [Google Scholar]
- Boespflug E. L., Storrs J. M., Allendorfer J. B., Lamy M., Eliassen J. C., Page S. (2011). Mean diffusivity as a potential diffusion tensor biomarker of motor rehabilitation after electrical stimulation incorporating task specific exercise in stroke: a pilot study. Brain Imaging Behav. 8 359–369 10.1007/s11682-011-9144-1 [DOI] [PubMed] [Google Scholar]
- Brott T., Adams H. P., Jr., Olinger C. P., Marler J. R., Barsan W. G., Biller J., et al. (1989). Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20 864–870 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- Carter A. R., Astafiev S. V., Lang C. E., Connor L. T., Rengachary J., Strube M. J. (2003). Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann. Neurol. 67 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Tang F. T., Chen H. C., Chung C. Y., Wong M. K. (2000). Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch. Phys. Med. Rehabil. 81 447–452 10.1053/mr.2000.3837 [DOI] [PubMed] [Google Scholar]
- Coupar F., Pollock A., Rowe P., Weir C., Langhorne P. (2012). Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin. Rehabil. 26 291–313 10.1177/0269215511420305 [DOI] [PubMed] [Google Scholar]
- Crafton K. R., Mark A. N., Cramer S. C. (2003). Improved understanding of cortical injury by incorporating measures of functional anatomy. Brain 126(Pt 7) 1650–1659 10.1093/brain/awg159 [DOI] [PubMed] [Google Scholar]
- Fallani F. D., Pichiorri F., Morone M., Molinari M., Cincotti F., Babiloni F., et al. (2013). Multiscale topological properties of functional brain networks during motor imagery after stroke. Neuroimage 83 438–449 10.1016/j.neuroimage.2013.06.039 [DOI] [PubMed] [Google Scholar]
- Koyama T., Marumoto K., Miyake H., Domen K. (2013). Relationship between diffusion tensor fractional anisotropy and motor outcome in patients with hemiparesis after corona radiata infarct. J. Stroke Cerebrovasc. Dis. 22 1355–1360 10.1016/j.jstrokecerebrovasdis.2013.02.017 [DOI] [PubMed] [Google Scholar]
- Koyama T., Tsuji M., Miyake H., Ohmura T., Domen K. (2012). Motor outcome for patients with acute intracerebral hemorrhage predicted using diffusion tensor imaging: an application of ordinal logistic modeling. J. Stroke Cerebrovasc. Dis. 21 704–711 10.1016/j.jstrokecerebrovasdis.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Kusano Y., Seguchi T., Horiuchi T., Kakizawa Y., Kobayashi T., Tanaka Y., et al. (2009). Prediction of functional outcome in acute cerebral hemorrhage using diffusion tensor imaging at 3T: a prospective study. AJNR Am. J. Neuroradiol. 30 1561–1565 10.3174/ajnr.A1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. C., Fu T., Wu C. Y., Wang Y. H., Liu J. S., Hsieh C. J., et al. (2010). Minimal detectable change and clinically important difference of the Stroke Impact Scale in stroke patients. Neurorehabil. Neural Repair 24 486–492 10.1177/1545968309356295 [DOI] [PubMed] [Google Scholar]
- Lindenberg R., Renga V., Zhu L. L., Betzler F., Alsop D., Schlaug G. (2010). Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 74 280–287 10.1212/WNL.0b013e3181ccc6d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R., Zhu L. L., Rüber T., Schlaug G. (2012). Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum. Brain Mapp. 33 1040–1051 10.1002/hbm.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. R., D’Arceuil H. E., Westmoreland S., He J., Duggan M., Gonzalez R. G., et al. (2007). Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke 38 138–145 10.1161/01.STR.0000252127.07428.9c [DOI] [PubMed] [Google Scholar]
- Mori S. (2006). Introduction to Diffusion Tensor Imaging 1st Edn. Boston, MA: Elsevier. [Google Scholar]
- Pineiro R., Pendlebury S. T., Smith S., Flitney D., Blamire A. M., Styles P., et al. (2000). Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke 31 672–679 10.1161/01.STR.31.3.672 [DOI] [PubMed] [Google Scholar]
- Sanford J., Moreland J., Swanson L. R., Stratford P. W., Gowland C. (1993). Reliability of the Fugl-Meyer assessment for testing motor-performance in patients following stroke. Phys. Ther. 73 447–454. [DOI] [PubMed] [Google Scholar]
- Schaechter J. D., Fricker Z. P., Perdue K. L., Helmer K. G., Vangel M. G., Greve D. N., et al. (2009). Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum. Brain Mapp. 30 3461–3474 10.1002/hbm.20770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemanck S. K., Kwakkel G., Post M. W., Kappelle L. J., Prevo A. J., et al. (2008). Impact of internal capsule lesions on outcome of motor hand function at one year post-stroke. J. Rehabil. Med. 40 96–101 10.2340/16501977-0130 [DOI] [PubMed] [Google Scholar]
- Shelton F. N., Reding M. J. (2001). Effect of lesion location on upper limb motor recovery after stroke. Stroke 32 107–112 10.1161/01.STR.32.1.107 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Jenkinson M., Woolrich M. W., Beckmann C. F., Behrens T. E., Johansen-Berg H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 S208–S219 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Song F., Zhang F., Yin D. Z., Hu Y. S., Fan M. X., Ni H. H., et al. (2012). Diffusion tensor imaging for predicting hand motor outcome in chronic stroke patients. J. Int. Med. Res. 40 126–33 10.1177/147323001204000113 [DOI] [PubMed] [Google Scholar]
- Song J., Young B. M., Nigogosyan Z., Walton L. M., Nair V. A., Grogan S. W., et al. (2014). Characterizing relationships of DTI, fMRI, and motor recovery in stroke rehabilitation utilizing brain-computer interface technology. Front. Neuroeng. 7:31 10.3389/fneng.2014.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. K., Sun S. W., Ju W. K., Lin S. J., Cross A. H., Neufeld A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20 1714–1722 10.1016/j.neuroimage.2003.07.005 [DOI] [PubMed] [Google Scholar]
- Song S. K., Sun S. W., Ramsbottom M. J., Chang C., Russell J., Cross A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17 1429–1436 10.1006/nimg.2002.1267 [DOI] [PubMed] [Google Scholar]
- Stewart A. L., Ware J. E. (1992). Measuring Functioning and Well-Being : the Medical Outcomes Study Approach. Durham: Duke University Press. xxiii, 449. [Google Scholar]
- Stinear C. (2010). Prediction of recovery of motor function after stroke. Lancet Neurol. 9 1228–1232 10.1016/S1474-4422(10)70247-7 [DOI] [PubMed] [Google Scholar]
- Stinear C. M., Barber P. A., Smale P. R., Coxon J. P., Fleming M. K., Byblow W. D. (2007). Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 130(Pt 1), 170–180. [DOI] [PubMed] [Google Scholar]
- Varkuti B., Guan C., Pan Y., Phua K. S., Ang K. K., Kuah C. W., et al. (2013). Resting state changes in functional connectivity correlate with movement recovery for BCI and robot-assisted upper-extremity training after stroke. Neurorehabil. Neural Repair 27 53–62 10.1177/1545968312445910 [DOI] [PubMed] [Google Scholar]
- Ward N. S., Brown M. M., Thompson A. J., Frackowiak R. S. (2003). Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain 126(Pt 6), 1430–1448 10.1093/brain/awg145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring D. J., Toosy A. T., Clark C. A., Parker G. J., Barker G. J., Miller D. H., et al. (2000). Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol. Neurosurg. Psychiatry 69 269–272 10.1136/jnnp.69.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. M., Nigogosyan Z., Remsik A., Walton L. M., Song J., Nair V. A., et al. (2014a). Changes in functional connectivity correlate with behavioral gains in stroke patients after therapy using a brain-computer interface device. Front. Neuroeng. 7:25 10.3389/fneng.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. M., Nigogosyan Z., Walton L. M., Song J., Nair V. A., Grogan S. W., et al. (2014b). Changes in functional brain organization and behavioral correlations after rehabilitative therapy using a brain-computer interface. Front. Neuroeng 7:26 10.3389/fneng.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Zhu C., Zhang Y., Chen H., Qin W., Wang M., et al. (2009). A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage 47 451–458 10.1016/j.neuroimage.2009.04.066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.