Abstract
The stembarks of Harungana madagascariensis were analyzed for their content of chemical constituents, antinutrients, vitamin levels, and in vitro antioxidant properties in two solvent systems. Phytochemical screening revealed higher levels of alkaloids, saponins, and flavonoids in the methanolic (MHM) extract than in the dichloromethane (DCM) extract. The methanolic extract had higher contents of minerals, vitamins, and antinutrients except K, vitamin B1, and phytic acid, respectively. Antioxidant potentials of the stembark extracts were assessed by the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity, metal chelating activity, and ferric reducing power. The methanolic extract showed a better antioxidant activity (IC50=87.66±0.97 μg/mL) in the DPPH system. The metal chelating activity was higher in the methanolic extract (92.4% at 20 mg/mL), but lower than the control ethylenediaminetetraacetic acid (EDTA). The methanolic extract also showed greater ferric reducing power and was richer in phenolics (132.24±0.61 mgGAE/g) and flavonoids (259.05±2.85 mgQE/g). Antinutrient analysis of the extracts indicated low levels of phytic acid, oxalates, and hydrocyanides below the lethal doses. The LD50 (i.p. mice) of the extracts showed relatively low toxicity in the range 1000–1414 mg/kg. These results support the ethnomedicinal uses of this plant in the treatment of diseases related to oxidative stress and suggest that consumption of H. madagascariensis is not harmful nutritively.
Key Words: : antioxidant activity, bioactive compounds, Harungana madagascariensis, mineral element, vitamins
Introduction
The use of and search for drugs and dietary supplements from plants have accelerated in recent years.1 This may be attributed, among others, to the health benefits derived from phytochemicals, whose uses in traditional medicine have been documented.2–4 Ethnobotanical information from the south eastern part of Nigeria5 has shown that Harungana madagascariensis (Lam. ex. poir) (family Hypericaceae) is an edible medicinal plant. The plant is native to Central Africa, Democratic Republic of Congo, Sudan, Ethiopia, Lesotho, and South Africa, and is a small- to medium-sized bushy tree, about 4–7 m in height. The bark is employed in the treatment of malaria,6 river blindness, ulcer, asthma, hepatitis, dysmenorrhea, and toothache.7 Besides being a rich fodder for goats and sheep, the leaves of H. madagascariensis are used for the treatment of chest pains and urogenital infections.8 Fruits are edible and are used as an abortive.9 The gummy sap is used as enema to treat enteritis leprosy, ring worm, and skin diseases.7 The stembark is also used for the treatment of malaria.10 Documented scientific studies indicate isolation of compounds like anthrones, anthraquinones, xanthones, flavonoids, and essential oils from this plant.11–13 Also, various parts of the plant have been reported to possess antiprotozoan activity,14 antifungal and antimicrobial activity,15–17 antioxidant and modulatory effect on cyclophosphamide-induced neurotoxicity,18 as well as acting as antioxidant markers in alloxan-induced diabetic and carrageenan-induced inflammatory disorders in rats. To our knowledge, the levels of minerals, vitamins, antinutrients, acute toxicity, and in vitro antioxidant activity of the stembark of H. madagascariensis have not been reported despite its widespread use.
In the present study, we evaluated the contents of mineral elements, vitamins, antinutrients, LD50, and in vitro antioxidant properties of the stembark of H. madagascariensis from Nigeria using standard analytical methods.
Materials and Methods
Plant material
The stembark of H. madagascariensis was collected from a forest edge in Ikono Local Government Area of Akwa Ibom State, Nigeria, in the month of December 2010. Plant identification, authentication, and specimen referencing were done by Dr. Margaret Bassey, a plant taxonomist in the Department of Botany and Ecological Studies, University of Uyo, Nigeria. A voucher specimen has been deposited at the Herbarium of the Department of Botany and Ecological studies.
Sample preparation
The dried plant stembark (870.0 g) was ground into powder using a laboratory hammer mill and macerated first with dichloromethane (1.8 L) at room temperature for 24 h and later with methanol (1.8 L) at room temperature for 24 h. This was followed by filtration of the resulting solution and evaporation of the solvent in vacuo to obtain the crude dichloromethane (DCM) and methanol (MHM) extracts, respectively.
All reagents used were of analytical grade, and all experiments were done in triplicate.
Phytochemical analysis
Phytochemical tests to identify the constituents of the extracts were carried out using standard procedures outlined by Harborne19 and Trease and Evans.20 Precisely, screening of alkaloids was carried out with Mayer's and Dragendroff's reagents, saponins by Frothing and Fehling's tests. Cardiac glycosides were detected by the Liebermann's and Keller-Killiani's tests, tannins by the ferric chloride test, and phlobatannins by the hydrochloric acid test. Flavonoids were detected by the magnesium metal/hydrochloric acid test, triterpenes by the chloroform/acetic anhydride/sulfuric acid test, and anthraquinones by the benzene/ammonia solution test.
Quantitative determination of alkaloids was by the method of Harborne,19 while saponin was by the method used by Obadoni and Ochuko.21
Mineral analysis
Mineral digestion was done following the method of Egan et al.22 The concentrations of magnesium, manganese, and iron were determined using an atomic absorption spectrophotometer (AAS Unicam 919) in conjunction with reference mineral standards from Unicam Limited, United Kingdom. The flame photometer was used for the determination of potassium concentration in the extracts, while phosphates were determined by the colorimetric method using the UV-visible spectrophotometer model DR 2010.
Antinutrient analysis
The composition of total and soluble oxalates was determined by the Dye method23 and hydrocyanic acid by the method of AOAC.24 Phytic acid was determined by the method of McCane and Widdowson,25 while tannin determination was by Van-Burden and Robinson.26
Animals
Albino Swiss mice (20–25 g) of either sex were obtained from the University of Uyo animal house. They were kept under standard conditions in the animal house and maintained on standard animal pellets and water ad libitum. The animals were handled in accordance with the National Institutes of Health (NIH) guidelines of the Care and Use of Laboratory animals. Permission and approval for animal studies were obtained from the College of Health Sciences Animal Ethics committee, University of Uyo.
Acute toxicity test
The median lethal dose (LD50) of the extract was estimated using albino mice by intraperitoneal (i.p.) route using the method of Lorke.27 This involved the administration of different doses of the extract to groups of three mice each. The animals were observed for manifestation of physical signs of toxicity such as writhing, decreased motor activity, decreased body/limb tone, decreased respiration, and death. The number of deaths in each group within 24 h was recorded. The LD50 was calculated as the geometrical means of the maximum dose producing 0% mortality and the minimum dose producing 100% mortality. That is
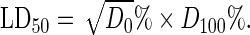 |
Vitamin analysis
The composition of water soluble vitamins, thiamine, and riboflavin was determined by the method of Scalar.28 Ascorbic acid content was obtained by the method of AOAC,24 while the vitamin A content was determined by spectrophotometry at 620 nm.
In vitro antioxidant analysis
In vitro antioxidant potentials of the extracts were determined using the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) assay,29 and the metal chelating activity was determined according to the method of Decker and Welch,30 while ferric reducing assay was by the method of Oyaizu.31 In all the measurements of antioxidant potentials, butylated hydroxyanisole (BHA), ethylenediaminetetraacetic acid (EDTA), and ascorbic acid were used as standards. The IC50 value was obtained by interpolation from linear regression analysis. Total phenolic content of the extracts was determined using the Folin–Ciocalteu reagent32 and expressed as mgGAE/g, while flavonoids were determined according to the method of Gulcin et al.33 and expressed as mgQE/g.
DPPH assay
In the DPPH assay, 1 mL of varying concentration of sample solutions was mixed with 1 mL of 0.004% methanol solution of DPPH. The mixture was shaken vigorously and allowed to stand for 30 min at room temperature in the dark. The reduction of the DPPH radical was determined by measuring the absorption at 517 nm. The procedure was repeated for the blank and control. The radical scavenging activity was calculated using the following equation.
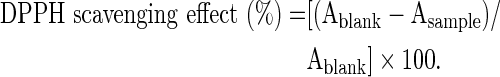 |
Extract concentration providing 50% inhibition (IC50) was calculated from the graph plotting inhibition percentage against extract concentration. BHA and ascorbic acid were used as positive controls.
Metal chelating activity
Metal chelating ability was determined according to the method of Decker and Welch,30 with some modifications. Precisely, 0.5 mL of sample was mixed with 0.05 mL of 2 mM FeCl2 and 0.1 mL of 5 mM ferrozine. The total volume was diluted with 2 mL methanol. Then, the mixture was shaken vigorously and left standing at room temperature for 10 min. After the mixture had reached equilibrium, the absorbance of the solution was measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine–Fe2+ complex formation was calculated using the following formula.
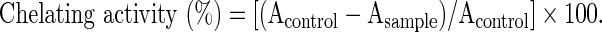 |
where Acontrol=absorbance of ferrrozine–Fe2+ complex and Asample=absorbance of sample. EDTA was used as a positive control.
Ferric reducing assay
The ferric reducing power was determined according to the method of Oyaizu.31 Each sample (0.1–20 mg/mL) in ethanol (2.5 mL) was mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.60) and 2.5 mL of 1% potassium ferricyanide and the mixture was incubated at 50°C for 20 min. After 2.5 mL of 10% trichloroacetic acid (g/mL) was added, the mixture was centrifuged at 200 g for 19 min. The upper layer (5 mL) was mixed with 5 mL of deionized water and 1 mL of 0.1% ferric chloride and the absorbance was measured at 700 nm against a blank. A higher absorbance indicated a higher reducing power. BHA was used as positive controls.
Results and Discussion
Phytochemical screening
Phytochemical screening of the dichloromethane and methanol stembark extracts of H. madagascariensis showed the presence of alkaloids, anthraquinones, flavonoids, cardiac glycosides, saponins, tannins, and terpenes. Alkaloids were absent in the dichloromethane extract, while phlobatannin was absent from the stembark. These results are given in Table 1. The presence of these phytochemicals in the stembark of H. madagascariensis makes it beneficial to the consumer as these compounds have potent medicinal values, including analgesic, antiplasmodial, bactericidal, wound healing, hypoglycemic, anti-inflammatory, and antioxidant properties, among others.34–36
Table 1.
Contents of Phytochemicals in Harungana madagascariensis Extracts
| Phytochemical | Test/reagents | DCM extract | MHM extract |
|---|---|---|---|
| Alkaloids | Dragendroff's | − | + |
| Mayer's | − | + | |
| Anthraquinones | Benzene/ammonia solution | + | + |
| Flavonoids | Magnesium metal, HCl acid | + | + |
| Cardiac glycosides | Liebermann's | + | + |
| Keller-Killiani's | + | + | |
| Phlobatannins | HCl acid solution | − | − |
| Saponins | Frothing | + | + |
| Fehling's tests | + | + | |
| Tannins | Ferric chloride solution | + | + |
| Terpenes | Chloroform, H2SO4 acid | + | + |
+, present; −, absent.
Quantitative determinations of alkaloids, flavonoids, and saponins showed that the methanol extract contained higher concentrations of these constituents than the dichloromethane extract (Table 2).
Table 2.
Quantitative Determination of Some Bioactive Compounds in H. madagascariensis Extracts
| Alkaloids | Saponins | |||
|---|---|---|---|---|
| Plant constituents | DCM | MHM | DCM | MHM |
| Concentration mg/100 g | 0.36±0.03 | 1.14±0.08 | 0.37±0.07 | 0.48±0.09 |
Alkaloids are efficient therapeutically significant plant substance. Pure, isolated, and synthetic derivatives are used as basic medicinal agents because of their analgesic, antiplasmodic, and bacterial properties.34 Saponins have been shown to possess both beneficial (cholesterol-lowering and hypoglycemic effects) and deleterious (cytotoxic and permeabilization of the intestine) properties.35 They also possess structure-dependent biological activities.36 Another important action of saponins is their expectorant action through the stimulation of a reflex of the upper digestive tract.37 A potent mix of flavonoids and alkaloids causes the production of more insulin in the body. Flavonoids are known for their anti-inflammatory and allergic effect coupled with their gastric mucus production. Flavonoids also possess some antibacterial and antifungal properties.38 It is possible that these bioactive compounds found in the stembark could be responsible for the various pharmacological activities of the plant and support its widespread use in traditional medicine.
Antinutrient composition
Levels of hydrogen cyanide, oxalate, phytic acid, and tannins in the dichloromethane and methanol extracts of H. madagascariensis stembark are given in Table 3.
Table 3.
Levels of Minerals, Vitamins, and Antinutrients in H. madagascariensis Extracts
| Dichloromethane extract (mg/100 gDW) | Methanol extract (mg/100 gDW) | |
|---|---|---|
| Fe | 2.03±0.01 | 2.50±0.02 |
| Mg | 13.52±0.04 | 25.99±0.04 |
| Mn | 20.33±0.03 | 29.44±0.03 |
| Phosphorus | 0.56±0.0 | 0.11±0.01 |
| K | 45.31±0.07 | 31.22±0.05 |
| Vitamin B2 | 10.14±0.03 | 18.22±0.03 |
| Vitamin B1 | 28.31±0.04 | 23.14±0.03 |
| Vitamin C | 0.16±0.01 | 0.26±0.01 |
| Vitamin A | 2.60±0.02 | 4.18±0.02 |
| Tannin | 0.51±0.01 | 0.97±0.02 |
| Phytic acid | 0.03±0.01 | 0.01±0.01 |
| Total oxalate | 3.64±0.02 | 4.63±0.04 |
| Soluble oxalate | 2.05±0.01 | 3.03±0.02 |
| Hydrocyanate | 0.54±0.01 | 0.75±0.02 |
| LD50 | 1000±8.0 mg/kg | 1414±8.2 mg/kg |
The content of hydrogen cyanide (0.75 mg/100 g) in the methanol extract was far below the lethal dose of 35 mg/100 g.39 Also, the quantity of soluble oxalates in the methanol extract (3.03+0.02 mg/100 gDW) was below the toxic level of 2–5 mg/100 g.40 Oxalates are known to bind to calcium to form calcium oxalate crystals, which are deposited as urinary calcium (stones) that are associated with blockage of renal tubules.41 Based on our obtained values, it could be speculated that the danger of toxicity arising from hydrogen cyanide or oxalate in the plant under study is low. Contents of phytic acid and tannins were found to be low in both extracts. Generally, the methanol extracts had slightly higher contents of these antinutrients than the dichloromethane extracts.
Acute toxicity test
The acute toxicity test showed that the administration of the methanol crude stembark extract at doses of 3000 and 2000 mg/kg resulted in 100% mortality of the animals in the various groups tested, while no death was recorded in the group administered with 1000 and 500 mg/kg of the sample. The LD50 (i.p. mice) value of 1414±8.2 mg/kg for MHM extract and 1000±8.0 mg/kg for DCM extract obtained indicated slight toxicity. In general, the smaller the LD50 value, the more toxic the sample is and the larger the LD50 value, the lower the toxicity. It therefore means that a large quantity of the material will be required to cause a toxic response.
Mineral elements
Obtained results indicate H. madagascariensis stembark to be a source of magnesium, manganese, potassium, phosphate, and iron (Table 3). The dichloromethane extract had higher contents of potassium and phosphorus than the methanol extract. Iwalewa et al.15 reported a higher value of 164 mg/g K for ethanol: water extract of H. madagascariensis. This variation may be attributed to the climate, soil structure, and extracting solvent. However, our values were lower than reports for potassium content in green vegetables (l32 mg/l00 g).42 Minerals have been reported to play significant roles in many health-promoting systems within the body,43,44 and thus, consumption of H. madagascariensis might play useful roles in optimizing their availability and utilization.
Vitamin composition
The vitamin content of H. madagascariensis stembark was higher in the methanol extract than the dichloromethane extract. Vitamins decreased in the following order: B1>B2>A>C. A trace amount of vitamin C was recorded (0.26+0.01 mg/100 gDW) in the methanol extract. Lee et al.45 have reported that edible plants possess chemopreventive and chemoprotective activities and these activities have been attributed to dietary constituents such as vitamin E, vitamin C, and more recently, polyphenols. Also, the wound healing of ascorbic acid has been reported,46 but the extremely low values obtained in this study do not entirely support its usage for wound healing, rather a reasonable justification could be deduced from a possible synergistic effect with tannins.
Antioxidant activity
In vitro antioxidant activity of methanol and dichloromethane stembark extracts of H. madagascariensis was determined using DPPH scavenging assay, metal chelating ability, and ferric reducing assay.
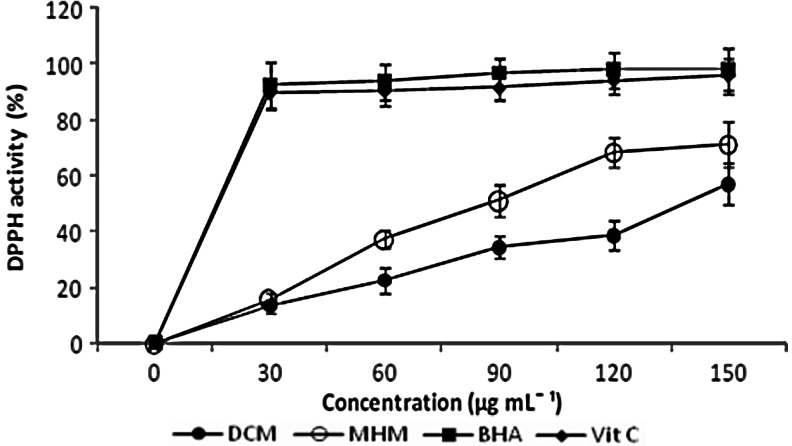
The DPPH assay as given in Figure 1 increased in a dose-dependent manner. The methanol extract exhibited a better radical scavenging ability (IC50=87.66+0.97 μg/mL) than the dichloromethane extract, even though the ability was lower than that observed for the standard BHA and ascorbic acid. The radical scavenging activity is extremely important due to the deleterious role of free radicals in living systems. Lower IC50 values indicate a higher radical scavenging activity.47 Our results are in agreement with reports from the purified water extracts of Kandelia candel and other medicinal plants of the Hypericaceae.48,49
FIG. 1.
DPPH activity of H. madagascariensis stembark extracts.
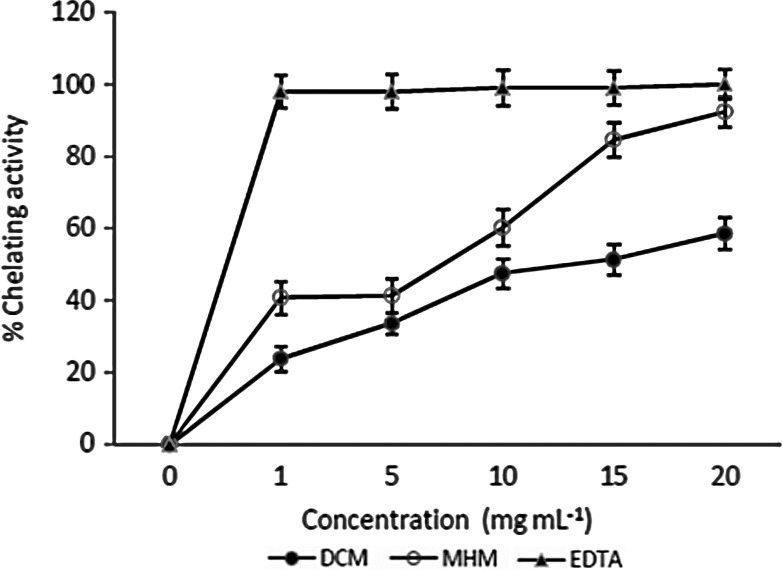
The metal chelating activity of the extracts is given in Figure 2. Result indicated a significant chelating ability, which increased with an increase in concentration. However, it was lower than that of the standard EDTA. The metal chelating activity indicates antioxidant and antiradical properties. Observed result indicated that the methanol extract is a better metal chelator than the dichloromethane extract.
FIG. 2.
Metal chelating activity of H. madagascariensis extracts.
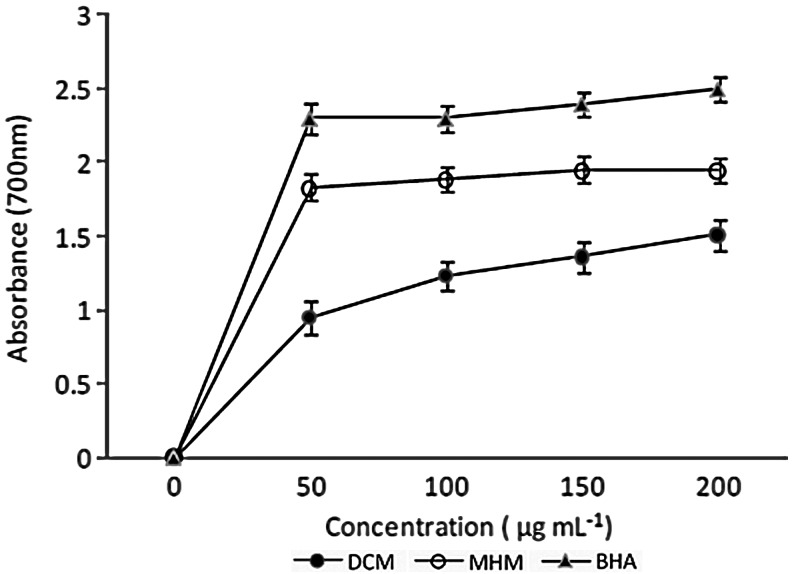
Reducing power of extract was determined by the ferric ion (Fe2+) reducing method (Fig. 3). The reducing ability of both extracts varied with varying concentrations. Generally, a higher absorbance indicates a greater reducing power. Obtained results indicated a higher reducing power in the methanol extract than the dichloromethane extract.
FIG. 3.
Reducing power of H. madagascariensis stembark extracts.
Flavonoid and phenolic contents of the extracts varied with solvent (Table 4). The methanol extract had higher contents of flavonoids (295.05±2.05 mgQE/g) and total phenolics (132.24±0.61 mgGAE/g) than the dichloromethane extract.
Table 4.
Total Phenolics, Flavonoid Content, and Antioxidant Activity of H. madagascariensis Extracts
| Extract | Total phenolics (mgGAE/g) | Flavonoid (mgQE/g) | DPPH assay IC50 (μg/mL) |
|---|---|---|---|
| Methanol | 132.24±0.61 | 259.05±2.05 | 87.66±0.97 |
| DCM | 35.5±0.52 | 250.02±0.75 | 37.52±0.13 |
| BHT | 16.24±0.15 | ||
| Ascorbic acid | 15.17±0.20 |
Phenolic compounds are secondary metabolites and are mostly represented by flavonoids and phenolic acids. Many studies have shown that phenolic compounds in plants show an antioxidant activity. These antioxidant compounds donate an electron to a free radical and convert it into an innocuous molecule.50 Flavonoids are polyphenolic compounds that have antioxidant properties and have been associated with protection against cancer and heart disease. Our result compared favorably with previous studies on extracts of family Hypericaceae and showed an equivalent or higher content of flavonoids and phenolics.49 Thus, the presence of significant amount of flavonoids and phenolics in the methanol extract may, in part, explain its antioxidant activity and metal chelating potential. This together with its content of minerals, vitamins, and phytochemicals lends credence to its various uses in ethnomedicine.
In conclusion, these analyses of H. madagascariensis methanol and dichloromethane stembark extracts showed that the plant contains some antioxidants and low levels of antinutrients—phytic acids, oxalates, and hydrocyanides. The plant, therefore, may play some contributory role in the prevention of diseases related to oxidative stress, in areas where it is widely consumed as herbs without any nutritive harm.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Balunas MJ, Kinghorn AD: Drug discovery from medicinal plants. Life Sci 2005;78:431–444 [DOI] [PubMed] [Google Scholar]
- 2.Choi SY, Lim SH, Kim JS, Ha TY, Kim SR, Kang KS, Hwang IK: Evaluation of the estrogenic and antioxidant activity of some edible and medicinal plants. Korean J Food Sci Technol 2005;37:549–556 [Google Scholar]
- 3.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A: Plant foods and herbal sources of resveratrol. J Agric Food Chem 2002;50:3337–3340 [DOI] [PubMed] [Google Scholar]
- 4.Pieroni A: Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J Ethnopharmacol 2000;70:235–273 [DOI] [PubMed] [Google Scholar]
- 5.Gill LS: Ethno Medicinal Uses of Plants in Nigeria. University of Benin Press, Benin, Nigeria, 1992, p. 130 [Google Scholar]
- 6.Kokwaro JO: Medicinal Plants of Africa. East African Literature Bureau, Nairobi, Kenya, 1976;51–54 [Google Scholar]
- 7.Abbiv DK: Useful Plants of Ghana. Intermediate Technology Publications and the Royal Botanic Garden, Kew, 1990, p. 52 [Google Scholar]
- 8.Maikere-Fanniyo R: Medicinal uses of Harungana madagascariensis (Lam. Ex. Poir). J Ethnopharmacol 1989;26:101–1092601351 [Google Scholar]
- 9.Vasileva B: Plante Medicinales De Guinea. Conakry, Republique De Guinea, 1990, p. 77 [Google Scholar]
- 10.Gessler MC, Haerdi F, Flemming A, Williams RO: Indigenous trees of Tanzania. Acta Trop 1994;56:65–778203297 [Google Scholar]
- 11.Linuma M, Hideki T, Tetsuro I, Toshiyuki T, Mohammad A: Two prenylated anthrones in Harungana madagascariensis. Phytochemistry 1995;40:267–271 [Google Scholar]
- 12.Simeon KF, Yapna DB, Krohn K, Ngadjui BT, Ngoupayo J, Choudhary MI, Schulz B: Antimicrobial prenylated anthraacene derivatives from the leaves of Harungana madagascariensis. J Nat Prod 2007;70:600–603 [DOI] [PubMed] [Google Scholar]
- 13.Kouam SF, Khan SN, Krohn K, Ngadjui BT, Kapche DF, Yapna DB, Seema Z, Moustafa A MY, Choudhary MI: Prenylated anthranoid antioxidants from the stem bark of Harungana madagascariensis. J Nat Prod 2005;66:1174–1179 [DOI] [PubMed] [Google Scholar]
- 14.Iwalewa EO, Adewale IO, Taiwo BJ, Arogundade T, Osinowo A, Daniyan OM, Adetogun GE: Effects of Harugana madascariensis stem bark extract on the antioxidant markers in alloxan induced diabetic and carrageenan induced inflammatory disorders in rats. J Complement Integr Med 2008;5:1–18 [Google Scholar]
- 15.Iwalewa EO, Suleiman MM, Mdee LK, Eloff JN: Antifungal and antibacterial activities of different extracts of Harungana madagascariensis stem bark. Pharm Biol 2009;47:878–885 [Google Scholar]
- 16.Moulari B, Pellequer Y, Chaumont JP, Guilaume YC, Millet T: In vitro antimicrobial activity of the leaf extract of Harungana madagascariensis lam. Ex. Poir(Hypericaceae) against strains causing otitis externa in dogs and cats. Acta Vet Hung 2007;55:97–105 [DOI] [PubMed] [Google Scholar]
- 17.Okoli AS, Okeke MI, Iroegbu CU, Ebo PU: Antibacterial activity of Harungana madagascariensis leaf extract. Phytother Res 2002;16:174–179 [DOI] [PubMed] [Google Scholar]
- 18.Oboh G, Akomolafe TL, Adefagha SA, Adetuyi AO: Antioxidant and modulatory effect of ethanolic extract of Madagascar harungana (Harungana madagascariensis) bark on cyclaphosphamide induced neurotoxicity in drugs. J Food Drug Anal 2010;18:171–179 [Google Scholar]
- 19.Harborne JB: Phytochemical Methods, 2nd Edition. Champman and Hall, London, 1984, p. 20–22 [Google Scholar]
- 20.Trease GE, Evans WC: Pharmacognosy. Thirteenth Edition. Balliere Tindall, London, 1989, p. 882 [Google Scholar]
- 21.Obadoni BO, Ochuko PO: Phytochemical studies and comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta states of Nigeria. Glob J Pure Appl Sci 2001;8b:203–208 [Google Scholar]
- 22.Egan H, Kirk RS, Sawyer R: Pearson's Chemical Analysis of Food. 8th Edn., Churchill Livingstone, Edinburg, United Kingdom, 1981, p. 24 [Google Scholar]
- 23.Dye WB: Studies on Halogeton globerulus. Weed 1956;4:55–60 [Google Scholar]
- 24.AOAC. Official Methods of Analysis. 13th Ed. Washington DC, New York, 1980, p. 55 [Google Scholar]
- 25.McCane RA, Widdowson EM: Phytin in human nutrition. Biochem J 1953;29:2694–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van-Burden TP, Robinson WC: Formation of complexes between protein and tannin acid. J Agric Food Chem 1981;1:77–78 [Google Scholar]
- 27.Lorke D: A new approach to practical acute toxicity testing. Arch Toxicol 1983;54:275–286 [DOI] [PubMed] [Google Scholar]
- 28.Scalar E: Segregated Flow Analyzer for Analytical Process. Laboratories, Netherland, 2000, pp. 45–61 [Google Scholar]
- 29.Blois MS: Anti-oxidant determinations by the use of a stable radical. Nature 1958;181:1199–1200 [Google Scholar]
- 30.Decker G, Welch D: An investigation into the mechanism of citrate-Fe2+-dependent lipid peroxidation. Free Radic Biol Med 1990;3:379–387 [DOI] [PubMed] [Google Scholar]
- 31.Oyaizu M: Studies on products of browning reactions: Antioxidative activities of browning reactions prepared from glucosamine. Jpn J Nutr 1986;44:307–315 [Google Scholar]
- 32.Lister E, Wilson P: Measurement of Total Phenolics and ABTS Assay for Antioxidant Activity. Crop Research Institute, Lincoln, New Zealand, 2001 [Google Scholar]
- 33.Gulcin I, Topal F, Ozturk-Sarikaya SB, Bursal E, Goren AC, Bilsel M: Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L). Rec Nat Prod 2011;5:158–175 [Google Scholar]
- 34.Stray F: The Natural Guide to Medicinal Herbs and Plants. Tiger Books International, London, 1998, pp. 12–16 [Google Scholar]
- 35.Mayasuki Y, Stoichi H: Extraction of blood glucose sugar lowering saponins from Beta vulgaris. Chem Abstr 1997;20:14–17 [Google Scholar]
- 36.Price KR, Johnson IT, Frederick GR: The chemical and biological significance on saponins in food in feeding stuff. CRC Crit Rev Food Sci Nutr 1997;5:56–59 [Google Scholar]
- 37.David H: The New Holistic Herbal. 3rd Edition. Findhorn press, USA, 1983, p. 241 [Google Scholar]
- 38.Akpan MM, Odeomena CS, Nwachukwu CN, Danladi B: Antimicrobial assessment of ethanolic extract of Costus afer leaves. Asian J Plant Sci Res 2012;2:335–341 [Google Scholar]
- 39.Munro A, Bassir O: Oxalate in Nigeria vegetables. W A J Biol Appl Chem 1969;12:14–17 [Google Scholar]
- 40.Blood DC, Radostits OM: Veterinary Medicine. 7th edition, Balliere Tindall, London, 1989, p. 589 [Google Scholar]
- 41.Banso A, Adeyemo SO: Evaluation of antimicrobial properties of tannins isolated from Dichrostachys cinerea. Afr J Biotechnol 2007;6:1785–1787 [Google Scholar]
- 42.Claude B, Paule S: The manual of natural living. 1st edition. Biddles Ltd., London, 1979, 98–101 [Google Scholar]
- 43.Aremu CY, Udoessien EI: Chemical estimation of some inorganic elements in selected fruits and vegetables. Food Chem 1989;37:227–234 [Google Scholar]
- 44.Gbolahan D: Lesson Note on Medical Importance of Trace Elements. Centre for Natural Health Studies, Surulere, Nigeria, 2001, p. 67 [Google Scholar]
- 45.Lee H, Jung EY, Suh HJ: Chemical composition and anti-stress effects of yeast hydrolysate. J Med Food 2009;12:1281–1285 [DOI] [PubMed] [Google Scholar]
- 46.Bursal E, Koksal E, Gulcin I, Goren AC: Antioxidant activity and polyphenol content of cherry stem (Ceraus avium L) determined by LC-MS/MS. Food Res Int 2013;51:66–74 [Google Scholar]
- 47.Wei S, Zhou H, Lin Y: Antioxidant activities of extracts and fractions from hypocotyls of mangrove plant Kandelia candel. Int J Mol Sci 2010;11:4080–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maskovic PZ, Mladenovic JD, Cvijovic MS, Dokovic GA, Solujic SR, Radojkovic MM: Phenolic content, antioxidant and antifungal activities of acetonic, ethanolic and petroleum ether extracts of Hypericum perforatum L. Hem Ind 2011;45:159–164 [Google Scholar]
- 49.Gulcin I, Mshvildadze V, Gepdiremen A, Elias R: Screening of antioxidant and antiradical activity of monodesmosides and crude extracts from Leontice smirnowii tuber. Phytomedicine 2006;13:343–351 [DOI] [PubMed] [Google Scholar]
- 50.El-Hela AA, Abdel-Hady NM, Dawoud TM, Abdo MH, Morsy TA: Phenolic content, antioxidant potential and Aedes Aegyptii ecological friend larvicidal activity of some selected Egyptian plants. J Egypt Soc Parasitol 2013;43:215–234 [DOI] [PubMed] [Google Scholar]