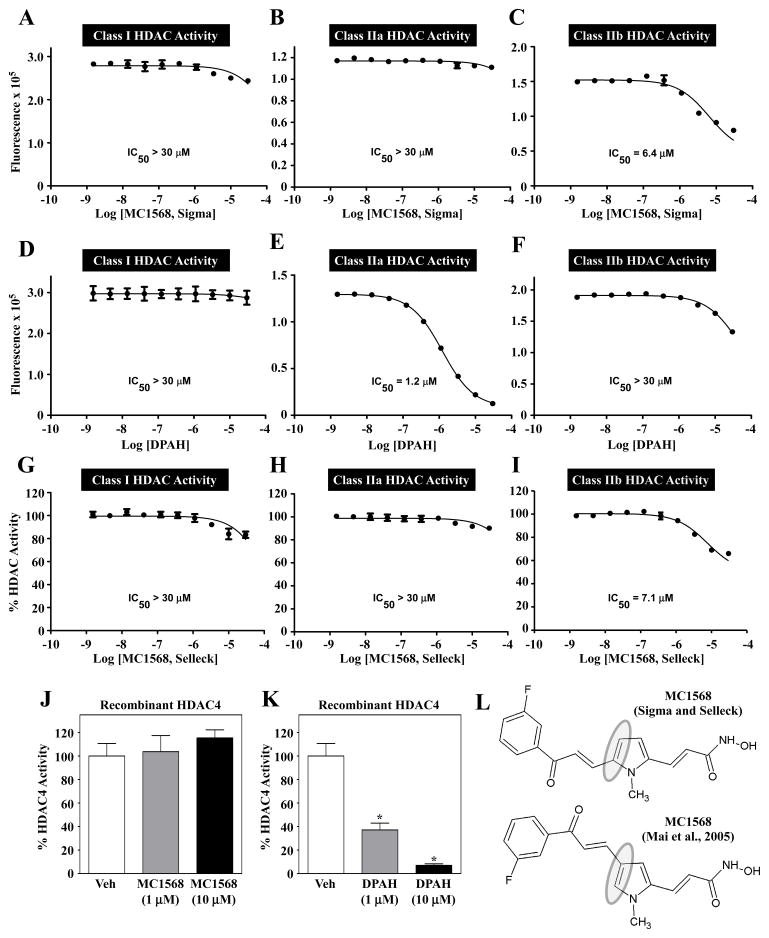
Fig. 2.
MC1568 does not block class IIa HDAC catalytic activity. Dose-response studies were performed to assess the ability of MC1568 and DPAH to inhibit HDAC catalytic activity in vitro. (A – C) MC1568 (from Sigma-Aldrich) failed to inhibit class I and class IIa HDAC activity, but did block class IIb activity at higher concentrations. (D – F) DPAH dose-dependently, and selectively, inhibited class IIa HDAC catalytic activity. MC1568 from Selleck Chemicals also failed to inhibit endogenous class IIa HDAC activity (G – I). MC1568 was unable to inhibit the catalytic activity of a recombinant form of a prototypical class IIa HDAC, HDAC4, while DPAH efficiently inhibited recombinant HDAC4 (J and K). (L) Analysis of NMR data from Sigma and Selleck revealed the commercial MC1568 to be a 2,5-disubstituted pyrrole, as opposed to the 2,4-disubstituted isomer shown in Fig. 1B.