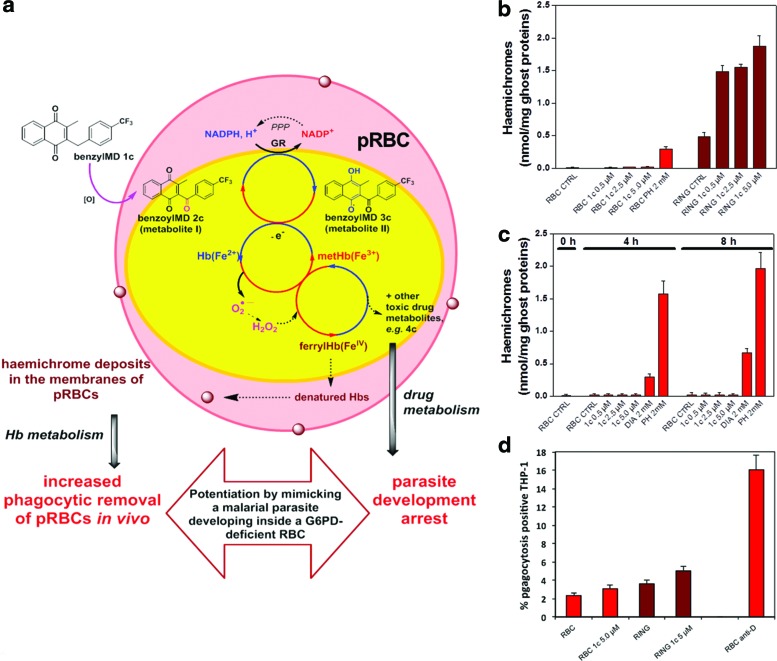
FIG. 6.
Potentiation of resistance of parasitized RBCs against malaria development: consequences of both drug metabolism and haemoglobin catabolism on (i) membrane-bound haemichromes in ring-pRBC versus non-parasitized G6PD-normal and non-parasitized G6PD-deficient RBCs, (ii) on phagocytosis by THP-1 cells of benzylMD 1c-treated or untreated ring-parasitized RBCs and non-parasitized RBCs. (a) Schematic figure of a pRBC treated by the benzylMD 1c: the lead drug is internalized in a pRBC and oxidized into the key metabolite, the benzoylMD 2c, which is reduced in a GR-catalyzed reaction in the cytosols of parasitized cells. The reduced benzoylMD 3c reduced metHb into Hb in a continuous NADPH-consuming redox cycle ending in ferrylHb formation and membrane-bound haemichromes. The benzylMD 1c at different concentrations was studied for its effects on G6PD-normal non-parasitized or ring-pRBCs. (b) Membrane-bound haemichromes in G6PD-normal non-parasitized RBCs (red bars), and ring-pRBCs (RING, wine bars) treated or not (CTRL) with the benzylMD 1c at 0.5 μM (10-fold IC50), 2.5 μM (50-fold IC50), 5.0 μM (100-fold IC50), or 2.0 mM PH, and incubated for 4 h at 37°C. Haemichromes are expressed as nmol/mg ghost protein; mean values±SD, n=2–4. Haemichromes were significantly higher (p<0.001) in treated ring-pRBCs compared with untreated ring-pRBCs and non-parasitized treated and untreated controls. (c) G6PD-deficient RBCs (hemizygous males, Mediterranean variant, G6PD activity<1% of normal enzyme). Membrane-bound haemichromes in non-parasitized G6PD-deficient RBCs, incubated during 4 and 8 h at 37°C without (CTRL) or with the benzylMD 1c at 0.5 μM (10-fold IC50), 2.5 μM (50-fold IC50), 5.0 μM (100-fold IC50), 2.0 mM DIA, or 2.0 mM PH. Haemichromes are expressed as nmol/mg ghost protein. Mean values of normal controls (mean±SD, n=2–4) are shown. Haemichromes were significantly higher (p<0.001) in DIA- and PH-treated G6PD-deficient RBCs compared to untreated controls. (d) Phagocytosis of ring-enriched pRBCs and non-parasitized RBCs treated or not with benzylMD 1c by THP-1 cells. After a 3 h incubation with benzylMD 1c at 5 μM (100-fold IC50) in a humidified CO2/air incubator, the cells (ring-enriched pRBCs [17–20% synchronized early rings enriched at 16–18 h after re-infection] and non-parasitized RBCs, treated or not with benzylMD 1c) were washed, opsonized with heterologous serum (0+group), and labeled with CFDA-SE (Sigma). CFDA-SE is a non-fluorescent lipophilic molecule that passively diffuses into the cell, where it is activated by esterase cleavage of its acetyl groups to the brightly fluorescent derivative CF-SE. CF-SE is a non-toxic molecule stably retained in the cell; it emits stable and homogeneous fluorescence and does not interfere with RBC functionality. THP-1 cells pre-stimulated during a 24 h incubation with tumor necrosis factor (250 U/ml) and interferon-gamma (50 U/ml) to enhance their phagocytic activity (21) were incubated with target RBCs for phagocytosis assay. After 2.5 h phagocytosis in a humidified CO2/air incubator, THP-1 cells were separated from non-phagocytosed RBCs and analyzed via flow cytometry. Opsonization with anti D-IgG was used as a positive phagocytosis control. Phagocytosis was expressed as a percentage of phagocytosis-positive THP-1 cells (mean values±SD, n=4). Phagocytosis of benzylMD 1c-treated ring-pRBC was significantly higher compared to phagocytosis of untreated ring-pRBCs (p=0.02) or benzylMD 1c-treated non-parasitized RBCs. (p=0.001). Increased phagocytosis of non-parasitized RBCs pre-treated with benzylMD 1c versus untreated non-parasitized RBCs was not significant. CFDA-SE, 5(6)-carboxyfluorescein diacetate N-succinimidyl ester; CTRL, drug-untreated control; DIA, diamide; PH, phenylhydrazine. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars