Abstract
The complement system has been shown to regulate T cell activation and alloimmune responses in graft-versus-host disease (GVHD). Mice deficient in the central component of complement system C3 have significantly lower GVHD-related mortality/morbidity and C3 modulates Th1/Th17 polarization in mouse GVHD. To investigate whether anti-complement therapy has any impact on human T cell activation, a drug candidate Compstatin was used to inhibit C3 activation in this study. We found the frequency of IFN-γ (Th1), IL-4 (Th2), IL-17 (Th17), IL-2 and TNF-α producing cells were significantly reduced among activated CD4+ cells in the presence of Compstatin. Compstatin treatment decreased the proliferation of both CD4+ and CD8+ T cells upon TCR stimulation. However, Compstatin does not affect the production of IL-2 and TNF-α in activated CD8+ T cells, and the differentiation of CD8+ T cells into distinct memory and effector subsets remained intact. Furthermore, we examined complement deposition in the skin and lip biopsy samples of patients diagnosed with cutaneous GVHD. C3 deposition was detected in the squamous epithelium and dermis, blood vessels and damaged sweat glands, and associated with gland damage and regeneration. We conclude that C3 mediates Th1/Th17 polarization in human T cell activation and skin GVHD in patients.
Keywords: Complement, GVHD, Compstatin, Th1, Th17
Introduction
Graft-verses-host disease (GVHD) is a major complication in allogeneic bone marrow transplantation (allo-HSCT), and characterized by epithelial cell injury in skin, intestine and liver (1–3). The development of GVHD involves donor T cell activation including proliferation, differentiation and inflammatory cytokine production, which lead to specific tissue damage (3–4). The interactions between the complement system and lymphocytes have been shown to regulate alloreactive T cell and APC function in the setting of allograft rejection (5–8). In recently published studies, we and others found complement proteins regulate GVHD in mouse models of bone marrow transplantation (9–10).
There are three pathways that activate the complement system: the alternative, lectin and classical pathways; all of which converge on the formation of the C3 convertase to propagate the complement cascade. Complement system has been shown to control CD4+ T cell activation and differentiation (5–6). Various CD4+ T cell subsets are essential regulators of immune responses, and Th1/2/17 polarization are reported to regulate GVHD (3, 11). Indeed, we demonstrated that reduced GVHD mortality/morbidity in C3-deficient mice is associated with a decrease in donor Th1/Th17 polarization (9).
Previous studies described complement deposition in GVHD tissues from both mouse and human (12–13). Furthermore, patients with sclerotic-type chronic GVHD (ScGVHD) have significantly elevated C3 in the serum (14). Given the emerging role of complement in alloimmune responses and T cell activation in animal models, it is important to address whether C3 modulates human T cell activation, polarization, expansion and differentiation. Compstatin is a 13-residue cyclic peptide that specifically binds to human C3 and inhibits complement activation, thus a favorable precursor peptide for the development of an anticomplement drug for oral use (15–16). Herein, we report that blocking C3 activation with Compstatin significantly inhibits Th1/Th17 polarization in activated human CD4+ T cells. The production of IL-2 and TNF-α are reduced in CD4+ but not in CD8+ T cells. Moreover, Compstatin treatment significantly decreases the proliferation of both CD4+ and CD8 +T cells. To evaluate the relevance of complement activation in human GVHD, we immunostained tissue samples, and found C3 deposition in the skin and lip biopsies of patients diagnosed with cutaneous GVHD.
Materials and Methods
Reagents
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat preparations derived from whole blood of healthy volunteer donors (Gulf Coast Regional Blood Center, Houston, TX). The buffy coat was diluted with PBS at 1:2 ratio and centrifuged over Histopaque-1077 (Sigma Diagnostics, St. Louis, MO) for 30 minutes. The PBMC interface was collected and analyzed for purity by flow cytometry and frozen for future use. Compstatin and peptide were purchased from R&D Systems (Minneapolis, MN). The hydrolyzed sodium powder was dissolved in DMSO and stored as recommended by the manufacturer.
Cytokine flow cytometry assay
Intracellular cytokine production was measured using 8-color 10-parameter cytokine flow cytometry as described (17). Briefly, 1 hour after simulation, Brefeldin A was added to enable accumulation of intracellular cytokines. Following 5 hours of incubation, cells were fixed and permeabilized with Fix & Perm A/B (Caltag, Burlingame, CA) and assessed for the simultaneous expression of surface markers and intracellular cytokines. FACS analyses were performed using mAbs for human CD4 (5 ug/ml), CD8 (5 ug/ml), IL-2 (2.5 ug/ml), IL-4 (2.5 ug/ml), IL-17 (2.5 ug/ml), TNF-α (1.5 ug/ml) and IFN-γ (1.5 ug/ml) (BD Pharmingen, San Jose, CA). After staining, cells were resuspended in PBS with 1% paraformaldehyde, then analyzed by an LSR-II cytometer (BD, San Jose, CA) and FlowJo software (Treestar, San Carlos, CA). At least 3x105 total events were analyzed with sequential gating of PBMCs in a lymphocyte region (by scatter) and on T cells (by assessing CD4+ or CD8+ staining). Gates defining cytokine-positive populations were based on the upper limits of fluorescence of unstimulated cells stained with the same antibodies.
T cell proliferation and phenotyping assay
PBMCs were stimulated with anti-CD3 mAb (OKT3) plus CD28, or mixed in a 1:1 ratio with irradiated allogeneic DCs in mixed lymphocyte reaction (MLR) as described (18). To determine cell proliferation, T cells were labeled with CFSE and cell division was monitored by using the FITC channel in a FACScanto II flow cytometer (BD Biosciences). Aliquots of PBMCs were analyzed by 7-color flow cytometry using a panel of surface molecule specific antibodies: CD4-APC (5 ug/ml), CD8-Pacific Blue (5 ug/ml), CD62L-APC/Cy7 (5 ug/ml), CD45RA-PE/Cy5 (2.5 ug/ml) and CD45RO-PE/Cy7 (2.5 ug/ml) (BD Pharmingen, San Jose, CA). Analyses of CD8+ T cell subsets were determined by assessing CD45RA and CD62L surface markers.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections were from biopsies retrieved from the GVHD tissue repository at Fred Hutchinson Cancer Center and the Dermatology Department at University of Texas Health Science Center at Houston. Anti-human C3 antibody (Abgent, San Diego, CA) was validated for use in immunohistochemistry on 3 formalin-fixed, paraffin-embedded human tissues. After incubation with the primary anti-human C3 antibody at 3 ug/ml, slides were incubated with MACH2TM detection kit (Biocare Medical, Concord, CA). The stained slides were evaluated by a pathologist to confirm staining specificity and photographed by light microscopy.
Results
Blocking C3 activation with Compstatin significantly reduces Th1/Th17 polarization
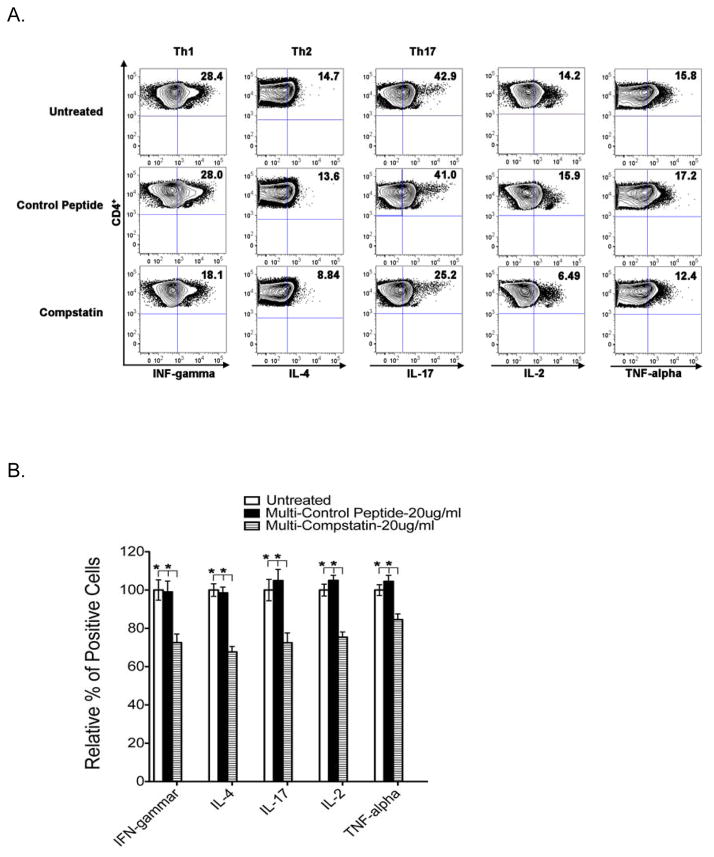
In the mouse model of GVHD, we have shown that the decreased mortality/morbidity in C3-deficient mice is associated with a decrease in donor Th1/Th17 polarization9. Using Compstatin that specifically binds to human C3 and inhibits complement activation, we further investigate the functional consequences of blocking C3 activation on human T cell activation. The production of IFN-γ (Th1), IL-4 (Th2), IL-17 (Th17), IL-2 and TNF-α was determined simultaneously in five normal donor samples to examine whether Compstatin affects T cell polarization upon activation. As shown in Figure 1 (A and B), the significantly reduced frequency of Th1 (18.1% vs. 28%), Th2 (8.84% vs. 13.6%), Th17 (25.2% vs. 41%), IL-2 (6.49% vs. 15.9%) and TNF-α (12.4% vs. 17.2%) producing cells was observed in CD4+ T cells treated with 20 μg/ml Compsatin in comparison to control peptide-treated samples. However, the frequency of IL-2 and TNF-α producing cells remained the same in CD8+ T cells (Figure 1C and 1D). Taken together, these results demonstrated that blocking C3 activation with Compstatin can inhibit Th1/Th17 polarization, and reduce IL-2 and TNF-α production only in activated CD4+ but not CD8+ T cells.
Figure 1. Multiple cytokine production in activated human T cells in the presence of Compstatin.
PBMCs from five donors were activated in the presence of 20 μg/ml Compstatin or the peptide control for 5–7 days. Intracellular cytokine production in CD4+ (A-B) and CD8+ (C-D) T cells was measured. (A, C) FACS were performed using mAbs for CD4, CD8, IFN-γ, IL-4, IL-17 (Th17), IL-2 and TNF-α. Representative dot plots were presented and the numbers in the quadrants represent the percentage of each population. Gates defining cytokine-positive populations herein were based on the upper limits of fluorescence of unstimulated cells stained with the same antibodies in Supplemental Figure 1. (B, D) Results are shown as bar graphs representing means ± S.D. of five independent donors normalized to that of untreated controls. P values were calculated using a t test comparing treated and untreated samples (* is for P < 0.05).
Blocking C3 activation with Compstatin significantly reduces human T cell proliferation
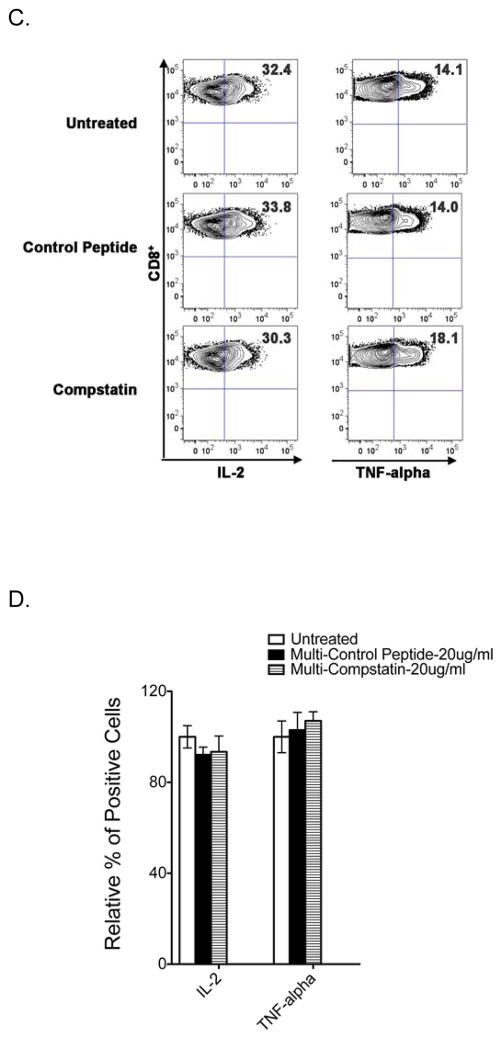
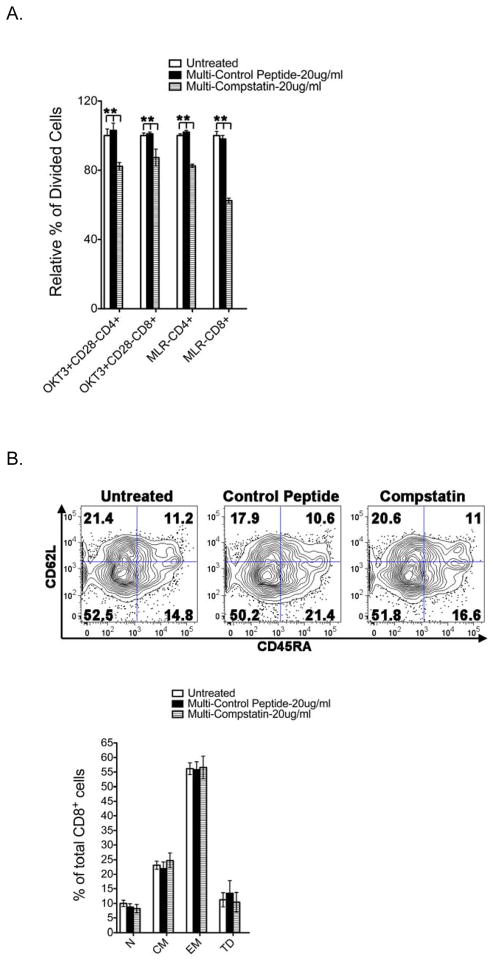
We examined the effect of Compstatin on the proliferation of both CD4+ and CD8+ T cells using CFSE-labeled cells stimulated with OKT3 plus CD28 or allogeneic DCs in MLR. As shown in Figure 2A, the frequency of divided cells was significantly reduced in the presence of Compstain at the concentration of 20 ug/ml. Upon OKT3 plus CD28 activation, the relative percentage of divided CD4+ and CD8+ T cells was decreased to 82.3% and 87.3% of untreated samples respectively. The reduction of divided CD4+ and CD8+ T cell was also observed in MLR with 82.6% and 62.4% of untreated samples respectively. Control peptide had no inhibitory effect on both CD4+ and CD8+ T cell proliferation. Thus, Compstatin inhibits the proliferation of CD4+ and CD8+ T cells upon TCR stimulation.
Figure 2. Effect of Compstatin on human T cell proliferation and differentiation.
PBMCs labeled with CFSE were activated for 5–7 days in the presence of 20 μg/ml Compstatin or the peptide control as indicated. (A) T cell proliferation was assessed by CFSE dye dilution following sequential gating on lymphocytes (by scatter) and CD4+ or CD8+ T cells. The percentage of divided CD4+ and CD8+ T cells were summarized from five independent donors activated by OKT3 plus CD28 or allogenic DCs in MLR. (B) Phenotype analysis of CD8+ T cell differentiation. A panel of surface antibodies including CD45RA and CD62L were used to determine naïve (N: CD45RA+ /CD62L+), central memory (CM: CD45RA− /CD62L+), effector memory (EM: CD45RA− /CD62L−), or terminal differentiated (TD: CD45RA+ /CD62L−) subset. Representative dot plots were presented and the numbers in the quadrants represent the percentage of each population. Results are shown as bar graphs representing means ± S.D. of five independent donors normalized to that of untreated controls. P values were calculated using a t test comparing treated and untreated samples (* is for P < 0.05).
We further examined the effect of Compstatin on CD8+ T cell differentiation upon stimulation in vitro. CD8+ T cells were classified into four distinct subsets based on CD45RA and CD62L expression: a naïve population, which is CD45RA+CD62L+; a central memory population, which is CD45RA−CD62L+; two effector memory populations, namely the CD45RA−CD62L− and the terminally differentiated CD45RA+CD62L− populations (19–21). As shown in Figure 2B, the activated CD8+ T cell compartment of untreated control samples consisted of 11.2% naïve (N), 21.4% central memory (CM), 52.5% effector memory (EM), and 14.8% terminally differentiated (TD) CD8+ T cells. Both Compstatin and control peptide did not alter the distribution of these four subsets, and thus Compstatin has no effect on CD8+ T cell differentiation upon TCR stimulation.
C3 deposition in human cutaneous GVHD tissues
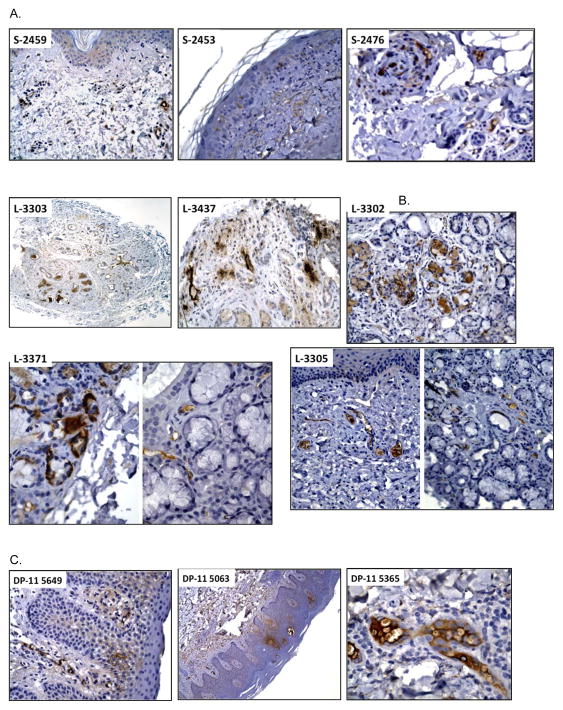
We examined the degree of C3 deposition in the skin and lip samples retrieved from our GVHD tissue repository of human allograft recipients. Total ten samples from skin and lip that showed histological features of GVHD were selected and stained with anti-human C3 antibody. C3 deposition were present in all slides examined. As shown in Figure 3A, representative example of skin GVHD tissues, C3 depositions were found in the squamous epithelium and dermis (S-2459, S-2453, S-2476), blood vessels (S-2459, S-2476) and damaged sweat glands (S-2476). Representative examples of the lip biopsy of GVHD patient are shown in Figure 3B, with damaged salivary gland which resulted in the dry mouth symptoms in patients (2, 18). C3 depositions were found in the lesion associated with gland damage and regeneration (L-3303, L-3437, L-3371). Furthermore, damaged blood vessels (L-3303, L-3302, L-3371, L-3305) were positive for C3 staining. In addition to the association with ScGVHD patients (14), C3 has been reported to play a role in skin inflammation in mice (22). Therefore, we examined C3 deposition in the skin lesion of patients with eczematous dermatitis and psoriasis (Figure 3C). In eczematous dermatitis, expression of C3 was found in the superficial papillary dermal capillary loops and focally in the spinous layer (DP-11 5649, DP-11 5063). Focal dermal staining with strong C3 deposition was found along the follicular infundibulum and in superficial papillary dermal capillary loops in psoriasis (DP-11 5365). Thus, C3 deposition is present in human cutaneous GVHD tissues, and inflamed skin from eczematous dermatitis and plaque patients.
Figure 3. C3 deposition in human cutaneous GVHD tissues and other skin disorders.
Formalin-fixed paraffin-embedded sections from patient biopsies were stained with anti-human C3 antibody. (A) Three representative skin biopsy samples from GVHD patients were shown: S-2459 (x200), S-2453 (x400), and S-2476 (x400). (B) Five representative lip biopsy samples from GVHD patients were shown: L-3303 (x50), L-3437 (x200), L3302 (x200), L-3371 (x400, two-separated area) and L-3305 (x200, two-separated area). (C) Three representative skin biopsy samples from patients with psoriasis and eczematous dermatitis were shown: DP-11 5649 (x200), DP-11 5063 (x50) and DP-11 5365 (x400).
Discussion
In the current study, we investigated the role of complement component C3 in human T cell activation and its involvement in patients with skin GVHD. We demonstrated that inhibiting C3 activation with Compstatin reduces cytokine production and Th1/Th17 polarization in activated CD4+ T cells. Although both CD4+ and CD8+ T cell proliferation decreased in the presence of Compstatin, the cytokine production and differentiation of CD8+ T cells remains intact. In addition, we found C3 deposition in the skin and lip biopsies of human GVHD patients, and the inflamed skin of dermatitis and psoriasis patients. Together, our data indicated that C3 and complement pathway mediate alloimmune responses in human GVHD.
During the last decade innate immunity has been shown to modulate adaptive immunity through the interaction between the complement system and lymphocytes activation (5–8). The central step of the complement cascade involves the cleavage of C3. Recently, we and others have established the role of C3 and other complement products in GVHD mouse models (9–10). To further investigate whether targeting complement system has potential therapeutic impact on GVHD, we utilized a drug candidate Compstatin, a peptide that specifically inhibits C3 activation (15–16). The decrease of Th1 and Th17 responses during human CD4+ T cell activation in the presence of Compstatin (Figure 1A and 1B) recapitulates our previous observation in the mouse model, and thus affirms the hypothesis and preclinical data that C3 modulates Th1/Th17 polarization and GVHD. In addition, we found that Compstatin does not appear to affect the production of IL-2 and TNF-α in activated human CD8+ T cells (Figure 1C and 1D). Furthermore, the differentiation of CD8+ T cells into distinct memory and effector subsets remained intact in the presence of Compstatin (Figure 2B). Previous studies report that CD8+ effector T cells are responsible for GVL while the proliferation and differentiation of CD4+ T cells contribute to GVHD (3, 23–26). Although the GVL effect was not evaluated in published mouse models (9–10), we could cautiously speculate that it might be possible to target complement pathway including blocking C3 activation to minimize GVHD without compromising the GVT effect in patients.
We found C3 depositions in all skin and lip GVHD tissues examined, and associated with damaged tissue and blood vessels (Figure 3A and 3B). The local production of complement proteins by APCs including DCs in the tissues has been reported to play an important role in immunoregulation (27–29). To determine whether C3 was produced by DCs resided in the skin (Langerhans cells), we stained the skin and lip biopsies with Langerhans DC marker CD1a (18). Although CD1a+ epidermal Langerhans cells were abundant in five samples examined, they were not associated with C3 staining (data not shown). Thus, C3 deposition in human skin GVHD tissues is associated with tissue damage and blood vessels but not with DCs.
In summary, our data provide preclinical evidence supporting that complement pathway plays important roles in regulating T cell activation and GVHD. Activation of the complement system is a major pathogenic event that drives various inflammatory responses in numerous diseases, thus a highly promising target for drug development for the treatment of currently intractable major human diseases. A number of drug candidates are rapidly emerging that are currently being investigated in disease models and clinical trials (30). Studies on complement system and GVHD will not only significantly advance our knowledge of GVHD but also define a new function of the complement system in health and diseases, and thus provide a rationale and pre-clinical data for using complement inhibitors as novel therapeutic interventions for GVHD.
Supplementary Material
Footnotes
Supplementary information is available at BMT's website.
Disclosure Statement: The work is supported in part by ACS grant RSG-08-183-01-LIB (Q.M.), NIH/NIAID grant 1R21AI101932 (Q.M. and V.A-K.) and CPRIT grant RP101374 (V.A-K.). D.L., R.C. R.P. and M.S. performed research. D.L., R.C., K.Y.T. and G.E.S. analyzed data. A.M.A., R.E.C. and V.A-K participated in discussions and wrote the paper. Q.M. designed the research, analyzed data and wrote the paper. We have no conflicts of interest to disclose.
References
- 1.Korngold R, Friedman TM. Murine models for graft-versus-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’s Hematopoietic Cell Transplantation. 3. Blackwell Publishing Ltd; Malden, MA, USA: 2004. pp. 353–368. [Google Scholar]
- 2.Sale GE, Shulman HM, Hackman RC. Pathology of hematopoietic cell transplantation. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’s Hematopoietic Cell Transplantation. 3. Blackwell Publishing Ltd; Malden, MA, USA: 2004. pp. 286–299. [Google Scholar]
- 3.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara JLM, Cooke KR, Teshima T. The Pathophysiology of graft-vs.-host disease. In: Ferrara JLM, Cooke KR, Deeg HJ, editors. Graft-vs-Host-Disease. 3. Marcel Dekker; New York, NY, USA: 2005. pp. 1–34. [Google Scholar]
- 5.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 6.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W, Medof ME, Heeger PS, Sacks S. Graft-derived complement as a mediator of transplant injury. Curr Opin Immunol. 2007;19:569–576. doi: 10.1016/j.coi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Ma Q, Li D, Nurieva R, Patenia R, Bassett R, Cao W, et al. Reduced GVHD in C3-deficient mice is associated with the decrease of donor Th1/Th17 differentiation. Biol Blood Marrow Transplant. 2012;18:1174–1181. doi: 10.1016/j.bbmt.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, et al. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–2238. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117:3268–3276. doi: 10.1182/blood-2010-12-290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niculescu F, Niculescu T, Nguyen P, Puliaev R, Papadimitriou JC, Gaspari A, et al. Both apoptosis and complement membrane attack complex deposition are major features of murine acute graft-vs.-host disease. Exp Mol Pathol. 2005;79:136–145. doi: 10.1016/j.yexmp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Tsoi MS, Storb R, Jones E, Weiden PL, Shulman H, Witherspoon R, et al. Deposition of IgM and complement at the dermoepidermal junction in acute and chronic cutaneous graft-vs-host disease in man. J Immunol. 1978;120:1485–1492. [PubMed] [Google Scholar]
- 14.Martires KJ, Baird K, Steinberg SM, Grkovic L, Joe GO, Williams KM, et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118:4250–4257. doi: 10.1182/blood-2011-04-350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morikis D, Assa-Munt N, Sahu A, Lambris JD. Solution structure of Compstatin, a potent complement inhibitor. Protein Sci. 1998;7:619–627. doi: 10.1002/pro.5560070311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson B, Larsson R, Hong J, Elgue G, Ekdahl KN, Sahu A, et al. Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation. Blood. 1998;92:1661–1667. [PubMed] [Google Scholar]
- 17.Li D, Li Y, Hernandez JA, Patenia R, Kim TK, Khalili J, et al. Lovastatin inhibits T-cell proliferation while preserving the cytolytic function of EBV, CMV, and MART-1-specific CTLs. J Immunother. 2010;33:975–982. doi: 10.1097/CJI.0b013e3181fb0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer LA, Sale GE, Balogun JI, Li D, Jones D, Molldrem JJ, et al. Chemokine receptor CCR5 mediates alloimmune responses in graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:311–319. doi: 10.1016/j.bbmt.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 21.Tussey L, Speller S, Gallimore A, Vessey R. Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol. 2000;30:1823–1829. doi: 10.1002/1521-4141(200007)30:7<1823::AID-IMMU1823>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Purwar R, Bäumer W, Niebuhr M, Tschernig T, Kietzmann M, Werfel T. A protective role of complement component 3 in T cell-mediated skin inflammation. Exp Dermatol. 2011;20:709–714. doi: 10.1111/j.1600-0625.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 23.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L− memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Joe G, Zhu J, Carroll R, Levine B, Hexner E, et al. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 27.Reis ES, Barbuto JA, Kohl J, Isaac L. Impaired dendritic cell differentiation and maturation in the absence of C3. Mol Immunol. 2008;45:1952–1962. doi: 10.1016/j.molimm.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Kerekes K, Cooper PD, Prechl J, Józsi M, Bajtay Z, Erdei A. Adjuvant effect of gamma-inulin is mediated by C3 fragments deposited on antigen-presenting cells. J Leukoc Biol. 2001;69:69–74. [PubMed] [Google Scholar]
- 29.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176:3330–3341. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2001;48:1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.