Figure 1.
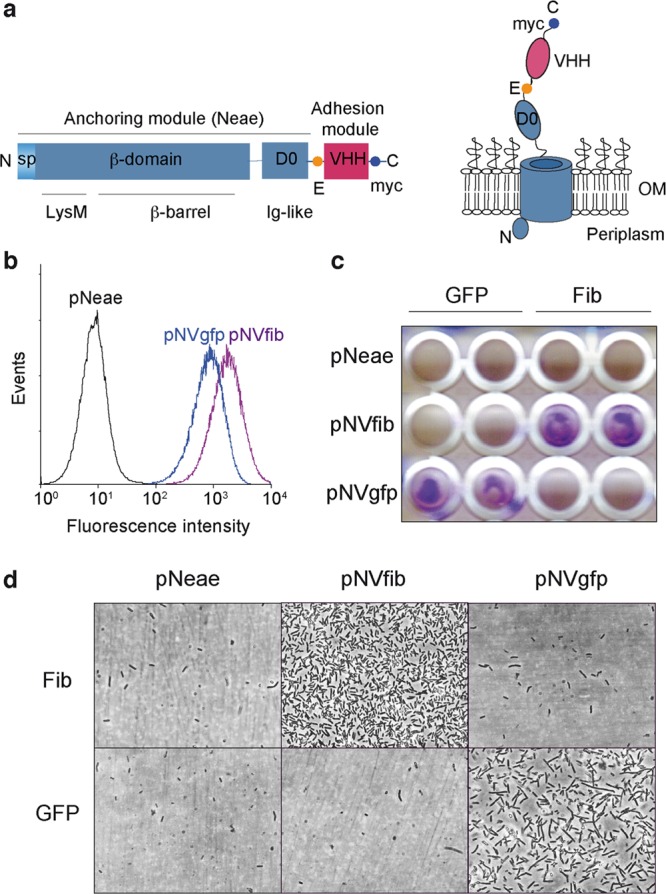
Synthetic adhesins and targeting of E. coli cells to antigens immobilized on a plastic surface. (a) Scheme of the primary structure of SAs (left) showing the N-terminal domain of intimin (Neae) as anchoring module, comprising the signal peptide (SP), LysM, β-barrel, and D0 Ig-like domains, fused to a variable Ig domain from heavy-chain-only antibodies (VHH) as adhesion module. The E-tag and myc-tag epitopes flanking the VHH domain are also indicated. Model of a SA fusion protein (right) in the bacterial outer membrane (OM) with the VHH exposed to the extracellular milieu. (b) Flow cytometry analysis of IPTG-induced E. coli EcM1 bearing pNeae (control), pNVgfp, or pNVfib plasmids. Histograms show the fluorescence intensity of bacteria stained with anti-myc mAb and secondary anti-mouse IgG-Alexa 488. (c) Induced E. coli EcM1 expressing the Neae polypeptide control (pNeae) or SAs against GFP (pNVgfp) or human fibrinogen (pNVfib) were incubated with plastic surfaces coated with GFP or human fibrinogen (Fib), as indicated. Bacterial adhesion was assessed by crystal violet staining. (d) Adhesion of E. coli bacteria to target antigen-coated plastic surface (as in panel c) observed under the light microscope.
