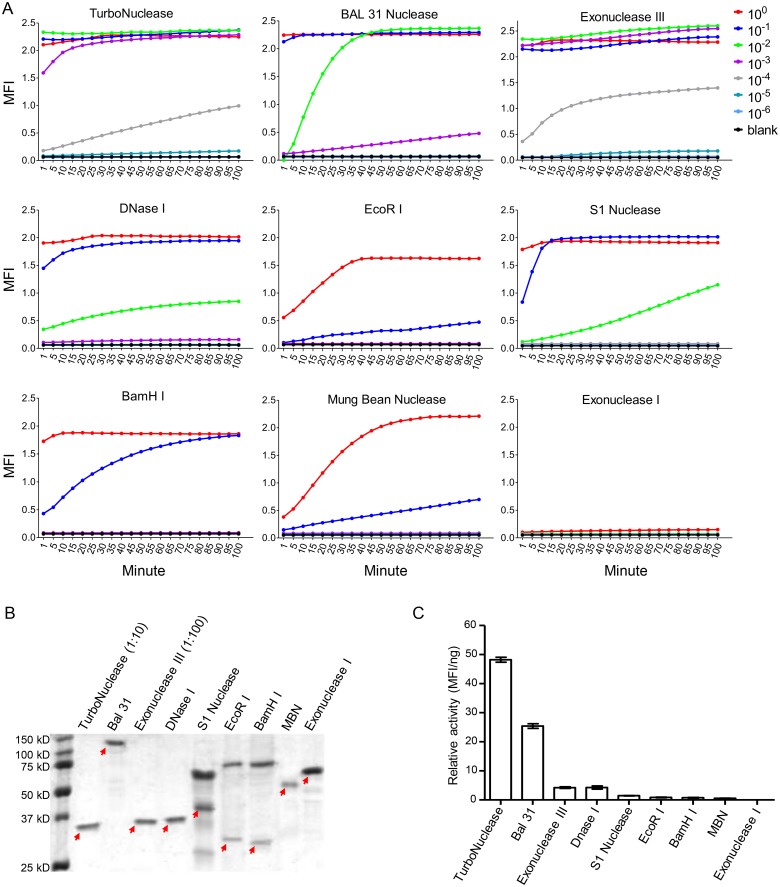
Fig 2. Determination of relative activity of different nucleases.
(A) The nuclease activity was determined by mixing FLOS with serially diluted enzymes (1:10). The mean fluorescence intensity (MFI) was measured every 5 minutes. (B) Analysis of the enzyme contents in the commercial enzyme stocks by SDS-PAGE. Equal volume (10 μl) of each commercial nuclease was loaded onto a SDS-PAGE gel for electrophoresis. Exonuclease III and TurboNuclese were diluted at 1:100 and 1:10, respectively, due to the high protein concentration in the samples. (C) Determination of enzyme relative activity. The relative activity of each enzyme was defined by the positive MFI (2.1 folds above the background) at the lowest concentration of the enzyme (MFI/ng). All experiments were independently performed three times and results from one representative experiment are shown. Negative control (NC) contained all components except the enzyme. Means ± SD are shown.