Abstract
Even the most rudimentary social cues may evoke affiliative responses in humans and promote social communication and cohesion. The present work tested whether such cues of an agent may also promote communicative interactions in a nonhuman primate species, by examining interaction-promoting behaviours in chimpanzees. Here, chimpanzees were tested during interactions with an interactive humanoid robot, which showed simple bodily movements and sent out calls. The results revealed that chimpanzees exhibited two types of interaction-promoting behaviours during relaxed or playful contexts. First, the chimpanzees showed prolonged active interest when they were imitated by the robot. Second, the subjects requested ‘social’ responses from the robot, i.e. by showing play invitations and offering toys or other objects. This study thus provides evidence that even rudimentary cues of a robotic agent may promote social interactions in chimpanzees, like in humans. Such simple and frequent social interactions most likely provided a foundation for sophisticated forms of affiliative communication to emerge.
Keywords: Communication, Interaction-promoting behaviours, Chimpanzees, Robot, Imitation
Introduction
In humans, the most rudimentary cues of others evoke affiliative behaviours, such as helping gestures or smiles, which may promote communicative exchanges and help initiate or maintain social cohesion in a variety of contexts (Dunbar et al. 2011; Ishii et al. 2011; Vogel 2010; Nadel et al. 2004). Humans even direct such behaviours towards interactive robots (Billard et al. 2006; Hiolle et al. 2012; Murray et al. 2009), agents with obvious limitations in appearance and actions compared to real individuals. The simplest of social cues produced in everyday situations may, thus, have an important impact on human communication and affiliation. The current study tested whether, like in humans, communicative interactions may be promoted in nonhuman primates by rudimentary cues of an agent, by examining chimpanzees (Pan troglodytes) during interactions with a robot.
While nonhuman primates also show a range of behaviours that promote affiliative interactions (Paukner et al. 2009; Sussman et al. 2005; Bard 2003; Gervais and Wilson 2005), it is still a research challenge to determine how readily such interactions may surface, as positive behaviours (e.g. play invitations) seem to be closely linked to meaningful social settings (Szameitat et al. 2009; Bard 1998; Davila Ross et al. 2008). Aversive behaviours, in contrast, seem to be evoked more readily, perhaps due to their strong links to survival (e.g. fight-or-flight reactions: Mobbs et al. 2007; see Fredrickson 2001), but they are clearly not used to uphold social encounters.
This study focused on a range of interaction-promoting behaviours in a nonhuman primate (Paukner et al. 2009; Davila-Ross et al. 2011): imitation, laughter, and response requests (behaviours that explicitly call for responses in others). Interaction-promoting behaviours may increase communicative exchanges among social partners. Experimental research on capuchin monkeys, for instance, revealed a strong association between imitation and affiliation, where the subjects preferred humans who imitated them over others (Paukner et al. 2009). One study indicated that great apes responded to such imitators with behaviours that tested the contingency of the social interactions, an apparent cognitively complex behaviour not observed in monkeys (Haun and Call 2008; also see Nielsen et al. 2005; Paukner et al. 2005). Furthermore, a study on chimpanzees during natural social play revealed laugh-induced laughter that was linked to longer play bouts (Davila-Ross et al. 2011).
The main goal of the present work was to examine how readily interaction-promoting behaviours may be evoked in chimpanzees. Specifically, it was tested in 16 chimpanzees if they directed interaction-promoting behaviours towards the robot, i.e. if they responded with active interest to imitation and laughter sent out by the robot, and if they requested responses from it during relaxed or positive contexts. Furthermore, if chimpanzees interact with a robot like with a social agent, it would validate the application of interactive robots to examine meaningful communicative behaviours within controlled settings in nonhuman primates. Whereas experimental research on nonhuman primates included either real social agents or no agents, the current study was markedly different. The humanoid appearance and simple actions of the robot resembled only to a minimal extent the cues of a real individual (Fig. 1a; also see Billard et al. 2006). Previous research involving nonhuman mammals and robots was primarily conducted to assess the application of robots (e.g. for domestic use) as well as the potential for future research (Gribovskiy 2011; Kubinyi et al. 2004; Laschi et al. 2006). These works did not include nonhuman primates.
Fig. 1.
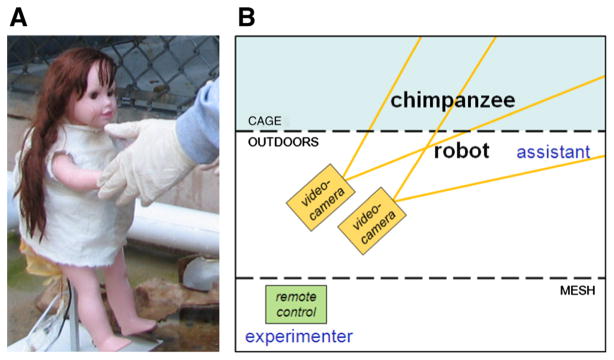
a The robot and b experimental setting. The robot was placed in front of the home cage of every subject. First, a human–robot interaction (with assistant) was shown to the subject, where the robot faced the assistant. Then, the robot was presented to the subject, to initiate the chimpanzee–robot interaction. Interactions were video-recorded. Robot movements and playbacks were remote-controlled by the experimenter
Materials and methods
Subjects
Subjects were 16 adolescent and adult chimpanzees (9 females), housed at the Yerkes National Primate Research Center (Emory University). All subjects were typically functioning and indicated some interest/curiosity after detecting the robot, by gazing at it. The robot was presented to 6 additional chimpanzees (4 females), but they were excluded from analyses (5 chimpanzees immediately avoided the robot for more than 4 min; one was behaviourally distressed for more than 4 min without a sign of calming down).
Robot
The interactive robot (Robota, Ecole Polytechnique Fédérale de Lausanne) was doll-shaped (Fig. 1a; height: 45 cm) and its movements resembled simple bodily actions. Its head could rotate (up to 90°; 3 stops, equally spaced: right, frontal, and left), each arm could lift and lower (up to 180°; 3 stops, equally spaced: straight above the head, at shoulder level, and along body), and each leg could lift and lower (up to 90°; 3 stops, equally spaced: from standing to hip level). The robot’s arms and legs could move independently. Sounds could be sent out from a small loudspeaker in its chest area, which was covered by a dress.
Set-up and data collection
The robot was placed in front of the chimpanzees’ home cages (Fig. 1b). Of the 16 subjects, 12 subjects were tested alone and 4 subjects were in pairs (3 pairs consisting of 2 subjects, 1 subject [the other chimpanzee was previously tested], and 1 subject [the other chimpanzee turned away; see ‘Subjects’], respectively). Subjects were paired when they were expected to be distressed for a long period of time if tested alone (based on JLR and JS’s research experience).
When seeing the robot, 14 subjects showed aversive behaviours (e.g. smashing boxes against a wall, piloerection), but 9 subjects started to calm down within the first minute. All subjects were calm prior to testing.
Fourteen of the subjects were tested in preset movement conditions and playback conditions (Table 1). For the pairs, the tested chimpanzees were predetermined. Movement conditions (imitation and no imitation) were compared to test whether the chimpanzees behaved differently as a function of being imitated by the robot. During imitation, the subjects’ head, arm, and leg movements were imitated by the robot. During no imitation, the robot moved the body parts either randomly or contingently (i.e. the chimpanzee and robot movements were in synchrony, but their body parts did not match, e.g. the chimpanzee turned the head and the robot lifted an arm). Seven subjects were tested during imitation, 6 during no imitation (4: random movements; 2: contingent movements). A male was excluded from the imitation analysis as he did not move.
Table 1.
Testing scheme for the study subjects
| Robot movement | Playbacks | Number of subjects |
|---|---|---|
| Imitation | Laughter always | 3* |
| Both laughter and screams | 3 | |
| Screams always | 2 | |
| No imitation | Laughter always | 3 |
| Both laughter and screams | 3 |
One subject (*) did not move and could, thus, not be included in the imitation analysis
Playback conditions (laughter and screams) were compared to test whether the chimpanzees responded to laughter sent out by the robot. Two presentations took place during the chimpanzee–robot interactions, i.e. 10–30 s after the robot was presented to the subjects (playback 1) and 2 min later (playback 2). Each playback lasted 5–8 s and included either two consecutive laugh sounds or two consecutive screams. The playback sounds were recorded from 8 unfamiliar juvenile and adult chimpanzees from a different facility (6 laughter and 7 scream recordings).
Testing began when the subjects were either facing the robot or sideways to it and were showing no sign of aggression (e.g. bluff displays with piloerection). The interaction ended when the subjects stopped responding to the robot (chimpanzee–robot interactions lasted >4 min, with one exception (minimum duration: 2 min 36 s; maximum duration: 6 min 36 s); mean duration: 4 min 59 s).
Prior to each chimpanzee–robot interaction, a human–robot interaction was shown to the subjects, involving a familiar assistant (Fig. 1b). It was important to give the chimpanzees the chance to see that the robot could interact before they started to interact with it themselves. Furthermore, this interaction allowed testing whether the chimpanzees responded differently when they interacted with the robot versus when a human interacted with the robot. During the human–robot interaction, the robot faced the assistant (1–2 metres away) and either imitated the assistant’s movements or showed random/contingent movements. The movement condition was kept the same across the human–robot and the chimpanzee–robot interactions. After the subjects gazed at the human–robot interaction with no sign of aggression for at least 15 s, the robot was presented to the chimpanzees (it was turned around to face them) and the assistant tilted her head downwards to avoid interfering with the testing. The human–robot interactions were short in duration to allow sufficient time to examine the chimpanzee–robot interactions; observations based on three chimpanzees showed that their interactions with the robot lasted only a few minutes (based on two subjects and one chimpanzee who immediately avoided the robot). JLR was the assistant for 13 subjects and JS for 3 subjects.
The robot movements and playbacks were controlled remotely by the experimenter, 4–7 metres and two cage mesh fences away from the chimpanzees. To remote control the robot movements and playbacks, the computer program MFC Robota 1.0.0.1 (Ecole Polytechnique Fédérale de Lausanne) was installed in a Dell Latitude D620 laptop. Each subject was video-recorded throughout the experimental session; a second camcorder was used to record the robot and assistant (Fig. 1b; Sony Handycam DCR-TRV19E).
Coding and further analyses
Interaction-promoting behaviours are likely to evoke communicative exchanges among social partners. They were coded here when the chimpanzees gazed at the robot from 3 metres or less (showing interest/curiosity) with relaxed/play expressions and without any signs of aggression. These behaviours included active interest (to test for responses to imitation and laughter) and response requests.
Active interest was coded when the chimpanzees showed animated body movements or expressions (e.g. playful up-and-down head movements). It indicated higher arousal than calm interest, which lacked animated movement or expressions. To test for responses to imitation, coding for active interest and calm interest took place during the first 4 min and the last 40 s of all chimpanzee–robot interactions and human–robot interactions, respectively. To test for responses to laughter, coding for active interest and calm interest took place within the first 10 s, following each playback onset. The percentage duration of active interest was then calculated by dividing the duration of active interest by the duration of active interest and calm interest, multiplied by one hundred.
Response requests were coded when the chimpanzee behaviour called for a response in others, typically found during social interactions of chimpanzees with conspecifics or humans. They were coded when the subjects were closest to the robot (at cage fence) during the chimpanzee–robot interactions.
In addition, gaze was continually coded as directed towards the robot, the assistant, or elsewhere. It was measured as percentage occurrence across 10-s intervals. Gaze is often used as an index of interest and/or curiosity. Gaze alternation is often used as a measure of social referencing (e.g. Russell et al. 1997). Gestures and vocal and facial expressions directed to the robot were also coded.
Active interest, calm interest, gaze, gestures, and expressions were recorded with the coder naïve about the movement conditions (subjects and robot were separately video-recorded). They were coded by a second observer for intercoder reliability testing (active interest and calm interest: kappa = 0.82, N = 14 subjects, 14 min; gaze: kappa = 0.81, N = 14, 14 min; expressions and gestures: kappa = 0.82, N = 14, 312 behaviours).
Since this study examined whether the chimpanzees showed any response requests, the presence of these behaviours was most critically examined. We included only data that were independently coded as well as agreed by two coders for further analysis. In addition, an inter-coder reliability test was conducted between one coder and a third coder (Kappa = 0.75, N = 7, 40 behaviours). For repeated comparisons, Hommel–Hochberg corrections were applied and α levels were adjusted.
Results
Interaction-promoting behaviours
Imitated subjects showed active interest for significantly longer than subjects who were not imitated during the chimpanzee–robot interactions (two-tailed Mann–Whitney U test; U = 7.5, N = 7+6 subjects, p = 0.036; Fig. 2). There was no indication that the subjects were affected already earlier by imitation as no difference was found across the two movement conditions during the human–robot interactions (Mann–Whitney U test with Hommel–Hochberg corrections; U = 23.0, N = 8+6 subjects, p = 0.880; Fig. 2). Furthermore, the imitated chimpanzees tended to show longer active interest during the chimpanzee–robot interactions than during the human–robot interactions (two-tailed Wilcoxon’s matched-pairs signed-ranks with Hommel–Hochberg corrections: z = −2.21, N = 7 subjects, p = 0.027; Fig. 2). The robot moved a mean of every 6 and 7 s during imitation and no imitation, respectively.
Fig. 2.

Active interest of chimpanzees across the movement conditions. The imitated subjects displayed active interest for significantly longer than the other subjects (two-tailed Mann–Whitney U test: U = 7.5, N = 7+6 subjects, p = 0.036). The imitated subjects also showed active interest for longer when imitated by the robot than when the robot imitated the assistant (Wilcoxon’s matched-pairs signed-ranks with Hommel–Hochberg corrections: z = −2.21, N = 7 subjects, p = 0.027). Total number of subjects is shown in brackets
The chimpanzees’ active interest following each playback was also assessed. No statistically significant difference was found in percentage duration of active interest when comparing the two playback conditions (two-tailed Mann–Whitney U test; U = 46.0, N = 14 subjects, p = 0.572).
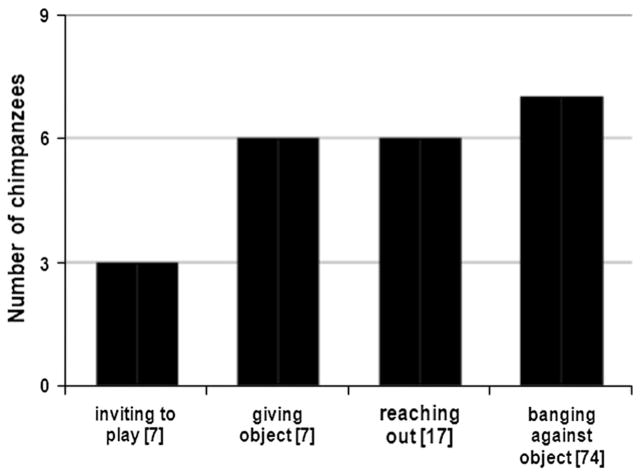
The chimpanzees directed four types of response requests towards the robot (Fig. 3). They invited the robot to play, gave the robot toys and other objects, reached out with their hands to the robot, and banged against objects. Although it is possible that the banging against objects represented a neo-phobic reaction, it is unlikely as the subjects were then calm and revealed no signs of aggression. It is more likely that banging was an attention-getting behaviour, similar to that used in interactions with humans (e.g. Hopkins et al. 2007; Leavens et al. 1996). Actions were not coded as response requests if the subjects acted in any way aggressively.
Fig. 3.
Chimpanzee response requests. Four types of response requests were directed to the robot. The occurrences of the requests are shown in brackets
Gaze, gestures, and expressions
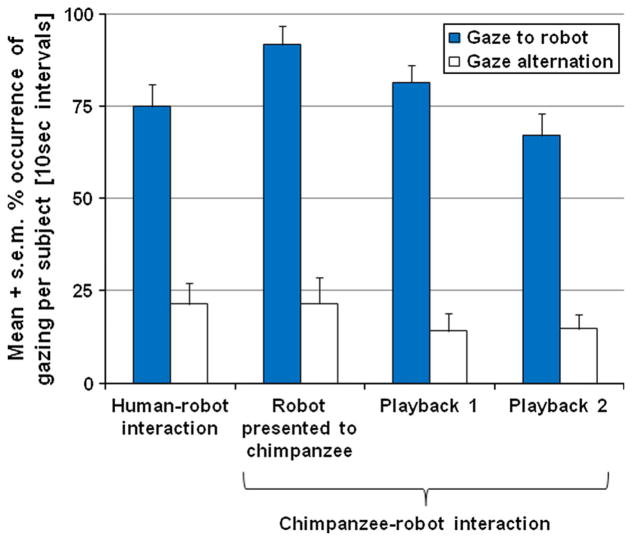
Overall, the chimpanzees (N ≤ 14 subjects) spent a mean of 79 % (s.e.m. = 4 %) of the 10-s intervals gazing at the robot (23 % gazing at the assistant). Occurrences of gaze at the robot changed significantly across the four periods of the experimental session (human–robot interaction, robot presented to chimpanzee, playback 1, and playback 2), with most gazes occurring once the chimpanzee–robot interaction started (repeated-measures ANOVA within-subject effect, F(3,33) = 4.50, p < 0.009, partial eta2 = 0.29, with a significant quadratic function (inverted U-shape, peaking during the robot-presented-to-chimpanzee period): F(1,11) = 8.61, p = 0.014, partial eta2 = 0.44; Fig. 4). Gaze to the robot did not differ as a function of imitation group (F(1,11) = 0.59, p = 0.460, partial eta2 = 0.05).
Fig. 4.
Gaze at the robot and gaze alternation. A significant difference was found for gaze at the robot across the four periods of this study (repeated-measures ANOVA within-subject effect: F(3,33) = 4.50, p < 0.009, partial eta2 = 0.29), but not for gaze alternations (repeated-measures ANOVA within-subject effect: F(2,20) = 0.55, p = 0.590, partial eta2 = 0.05)
The chimpanzees exhibited gaze alternations between assistant and robot a mean of 20 % the time (s.e.m. = 3.7 %) and at least once per subject (range from 1 to 15 10-s intervals), even though the assistants avoided interacting with the robot and the chimpanzee as much as possible. No significant change was found in gaze alternations across the four periods of the session (repeated-measures ANOVA within-subject effect: F(2,20) = 0.55, p = 0.590, partial eta2 = 0.05; Fig. 4).
During the chimpanzee–robot interactions, the chimpanzees (N = 16 subjects) directed a total of 258 gestures (N = 12), 37 vocalizations (N = 6), and 17 facial expressions (N = 5) to the robot. Gestures included reaching out the hand, waving the arms, cage banging, clapping, object offering, pressing the stomach to mesh, squeezing the lips through mesh, and throwing objects towards the robot; expressions included play faces, bared-teeth displays, raspberries, barks, cough grunts, hoots, and whimpers.
Discussion
The current study provides strong evidence that chimpanzees, like humans, respond with interaction-promoting behaviours to even the most rudimentary cues of an agent. The chimpanzees showed prolonged active interest when imitated by the interactive robot, and they requested responses from it in distinctive ways (for instance, by inviting play and offering toys). The chimpanzees did not show these behaviours towards the humans involved in the testing nor did they direct them elsewhere. The simple ways of inducing these behaviours by a robot suggest that social interactions of relaxed/playful contexts may readily surface among chimpanzees. Consequently, the present work indicates that opportunities for affiliative interactions frequently occur during everyday situations in chimpanzees and that such interactions play a highly significant role in social communication of these nonhuman primates.
The chimpanzees recognized being imitated by the robot. It is unlikely that the subjects’ responses to imitation were the outcome of signals inadvertently given by the assistant (the human most visible to the subjects). If such signals were given, they should have occurred prior to the chimpanzee–robot interactions, when the assistant was still actively involved. There was, however, no indication that imitation affected the subjects already at that time. Furthermore, the robot’s movement rates, controlled by the experimenter, were similar across the two conditions (every 6–7 s). Therefore, we conclude that chimpanzees must be highly susceptible to imitations, to an extent that they do not even require a real social partner. These findings concur with previous demonstrations that nonhuman primates recognize imitations by humans (Haun and Call 2008; Nielsen et al. 2005) and respond with affiliative behaviours (Paukner et al. 2009).
The chimpanzees predominantly gazed at the robot throughout the experimental session, indicating high interest/curiosity, and they also alternated gaze, perhaps to seek information from the assistant about this ambiguous agent (for research on social referencing in young chimpanzees, see Russell et al. 1997). As a related topic, their interest/curiosity (gaze) and animated behaviours (active interest in imitations) increased after the robot was turned away from the assistant and presented to them. Furthermore, the chimpanzees directed to the robot various species-typical gestures, vocalizations, and facial expressions as if it was a social agent (Goodall 1986; van Hooff 1973). Robotics research, thus, exhibits strong potential for offering a tool to future behaviour studies on nonhuman primates, particularly to examine communicative responses and interactions within a controlled and meaningful social setting.
One chimpanzee laughed during a play invitation, a vocalization which chimpanzees produce when they play with conspecifics (Davila-Ross et al. 2011). Despite such positive behaviours directed by the chimpanzees towards the robot, there was no indication that they responded with interaction-promoting behaviours to the laughter sent out by the robot. Although the samples limit generalizations, it is important to note that the outcomes concur with acoustic playback findings by providing no indication that chimpanzees respond positively upon merely hearing laughter [infants: Berntson et al. 1989; two zoo colonies: M. Davila-Ross, unpublished data]. Perhaps a real and familiar social partner and the natural playful context must be present for chimpanzee laughter to induce positive responses in conspecifics, as found in natural social play of chimpanzees (Davila-Ross et al. 2011). By contrast, human laughter may evoke positive behaviours via purely auditory means (Provine 1992), possibly due to the human-specific traits in laugh acoustics (e.g. regular voicing: Davila Ross et al. 2009; Bachorowski et al. 2001) or human-specific neural processes (Meyer et al. 2007).
In conclusion, the findings of the present work reveal that the simplest forms of social scenarios trigger positively grounded interactions in chimpanzees. Moreover, chimpanzees recognize when they are being imitated, even when imitation consists of movements by a robotic doll. Such simple social interactions have most likely provided a foundation for more complex forms of affiliative behaviours to emerge (see Bard et al. accepted; Bard et al. 2013; Boesch 2012; Gervais and Wilson 2005; Moll and Tomasello 2007; Tomasello and Hamann 2012).
Acknowledgments
We thank Florent D’halluin for technical assistance and M. Haas and D. Hartmann for the coding and discussions about this project. This study was funded by the European Commission’s FEELIX GROWING project (EC-FP6-IST-045169) and NIH grants NS-42867 and HD-60563. Research was conducted at Yerkes National Primate Research Center, Emory University. Playback recordings were obtained by MDR at Chimfunshi Wildlife Orphanage and Allwetterzoo Mϋnster. MDR and KAB designed the study; AB provided the robot; MDR, JLR, JS, and WHD conducted the study; JH, MDR, and KAB coded the observations; MDR, with assistance by KAB, analysed the data and wrote the paper.
Contributor Information
Marina Davila-Ross, Email: marina.davila-ross@port.ac.uk, Centre for Comparative and Evolutionary Psychology, Psychology Department, University of Portsmouth, Portsmouth, UK.
Johanna Hutchinson, Centre for Comparative and Evolutionary Psychology, Psychology Department, University of Portsmouth, Portsmouth, UK.
Jamie L. Russell, Division of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA, USA. Neuroscience Institute and Language Research Center, Georgia State University, Atlanta, GA, USA
Jennifer Schaeffer, Division of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA, USA.
Aude Billard, LASA Laboratory, School of Engineering, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland.
William D. Hopkins, Division of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA, USA. Neuroscience Institute and Language Research Center, Georgia State University, Atlanta, GA, USA
Kim A. Bard, Centre for Comparative and Evolutionary Psychology, Psychology Department, University of Portsmouth, Portsmouth, UK
References
- Bachorowski J-A, Smoski MJ, Owren MJ. The acoustic features of human laughter. J Acoust Soc Am. 2001;110:1581–1597. doi: 10.1121/1.2932088. [DOI] [PubMed] [Google Scholar]
- Bard KA. Social-experiential contributions to imitation and emotion in chimpanzees. In: Braten S, editor. Intersubjective communication and emotion in early ontogeny: a source book. Cambridge University Press; Cambridge: 1998. pp. 208–227. [Google Scholar]
- Bard KA. Development of emotional expressions in chimpanzees (Pan troglodytes) In: Ekman P, Campos JJ, Davidson RJ, de Waal FBM, editors. Emotions inside out: 130 years after Darwin’s the expression of the emotions in man and animals. Annals of the New York Academy of Sciences; New York: 2003. pp. 88–90. [DOI] [PubMed] [Google Scholar]
- Bard KA, Bakeman R, Boysen ST, Leaves DA. Emotional engagements predict and enhance social cognition in young chimpanzees. Dev Sci. 2013 doi: 10.1111/desc.12145. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard KA, Dunbar S, Maguire-Herring V, Veira Y, Hayes KG, McDonald K. Gestures and social-emotional communicative development in chimpanzee infants. Am J Primatol. 2013;9999:1–16. doi: 10.1002/ajp.22189. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Boysen ST, Bauer HR, Torello MS. Conspecific screams and laughter: cardiac and behavioral reactions of infant chimpanzees. Dev Psychobiol. 1989;22:771–787. doi: 10.1002/dev.420220803. [DOI] [PubMed] [Google Scholar]
- Billard A, Robins B, Dautenhahn K, Nadel J. Building Robota, a mini-humanoid robot for the rehabilitation of children with autism. Assist Technol. 2006;19:37–49. doi: 10.1080/10400435.2007.10131864. [DOI] [PubMed] [Google Scholar]
- Boesch C. The ecology and evolution of social behavior and cognition in primates. In: Vonk J, Shackelford T, editors. The Oxford handbook of comparative evolutionary psychology. Oxford University Press; Oxford: 2012. pp. 486–503. [Google Scholar]
- Davila Ross M, Menzler S, Zimmermann E. Rapid facial mimicry in orangutan play. Biol Lett. 2008;4:27–30. doi: 10.1098/rsbl2007.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila Ross M, Owren MJ, Zimmermann E. Reconstructing the evolution of laughter in great apes and humans. Curr Biol. 2009;19:1106–1111. doi: 10.1016/j.cub.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Davila-Ross M, Allcock B, Thomas C, Bard KA. Aping expressions? Chimpanzees produce distinct laugh types when responding to laughter of others. Emotion. 2011;11:1113–1120. doi: 10.1037/a0022594. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Baron R, Frangou A, et al. Social laughter is correlated with elevated pain threshold. Proc R Soc B. 2011;279:1161–1167. doi: 10.1098/rspb2011.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology: the broaden-and-build theory of positive emotions. Am Psychol. 2001;56:218–226. doi: 10.1037/0003-066X.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais M, Wilson DS. The evolution and functions of laughter and humor: a synthetic approach. Q Rev Biol. 2005;80:395–430. doi: 10.1086/498281. [DOI] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Harvard University Press; Cambridge: 1986. [Google Scholar]
- Gribovskiy A. Dissertation. Ecole Polytechnique Fédérale de Lausanne; 2011. Animal-robot interaction for ethological studies: An advances framework based on socially integrated mobile robots. [Google Scholar]
- Haun DBM, Call J. Imitation recognition in great apes. Curr Biol. 2008;18:288–290. doi: 10.1016/j.cub.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Hiolle A, Canamero L, Davila-Ross M, Bard K. Eliciting caregiving behaviour in dyadic human-robot attachment-like interactions. Trans Interact Intell Syst. 2012;22:1–24. doi: 10.1145/2133366.2133369. [DOI] [Google Scholar]
- Hopkins WD, Taglialatela J, Leavens DA. Chimpanzees differentially produce novel vocalizations to capture the attention of a human. Anim Behav. 2007;73:281–286. doi: 10.1016/j.anbehav.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Miyamoto Y, Mayama K, Niedenthal PM. When your smile fades away: cultural differences in sensitivity to the disappearance of smiles. Soc Psychol Pers Sci. 2011;2:516–522. doi: 10.1177/1948550611399153. [DOI] [Google Scholar]
- Kubinyi AE, Miklósi A, Kaplan F, Gácsi M, Topál J, Csányi V. Social behavior of dogs encountering AIBO, an animal-like robot in a neutral and in a feeding situation. Behav Process. 2004;65:239–321. doi: 10.1016/j.beproc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Laschi C, Mazzolai B, Patanè F, et al. Design and development of a legged rat robot for studying animal-robot interaction. Bio Rob. 2006 doi: 10.1109/BIOROB.2006.1639160. [DOI] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) J Comp Psychol. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Baumann S, Wildgruber D, Alter K. How the brain laughs. Comparative evidence from behavioral, electrophysiological and neuroimaging studies in human and monkey. Behav Brain Res. 2007;182:245–260. doi: 10.1016/j.bbr.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll H, Tomasello M. Cooperation and human cognition: the Vygotskian intelligence hypothesis. Philos T Roy Soc B. 2007;362:639–648. doi: 10.1098/rstb2006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JC, Canamero L, Bard KA, Davila Ross M, Thorsteinsson K. The influence of social interaction on the perception of emotional expression: a case study with a robot head. Lect Notes Comput Sci. 2009;5744:63–72. doi: 10.1007/978-3-642-03983-6_10. [DOI] [Google Scholar]
- Nadel J, Revel A, Andry P, Gaussier P. Toward communication: first imitations in infants, low-functioning children with autism and robots. Interact Stud. 2004;5:45–74. doi: 10.1075/is.5.1.04nad. [DOI] [Google Scholar]
- Nielsen M, Collier-Baker E, Davis JM, Suddendorf T. Imitation recognition in a captive chimpanzee (Pan troglodytes) Anim Cogn. 2005;8:31–36. doi: 10.1007/s10071-004-0232-0. [DOI] [PubMed] [Google Scholar]
- Paukner A, Anderson JR, Borelli E, Visalberghi E, Ferrari PF. Macaques (Macaca nemestrina) recognize when they are being imitated. Biol Lett. 2005;1:219–222. doi: 10.1098/rsbl.2004.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A, Suomi SJ, Visalberghi E, Ferrari PF. Capuchin monkeys display affiliation toward humans who imitate them. Science. 2009;325:880–883. doi: 10.1126/science.1176269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine RR. Contagious laughter: laughter is a sufficient stimulus for laughs and smiles. B Psychonomic Soc. 1992;30:1–4. [Google Scholar]
- Russell CL, Bard KA, Adamson LB. Social referencing by young chimpanzees (Pan troglodytes) J Comp Psychol. 1997;111:185–193. doi: 10.1037//0735-7036.111.2.185. [DOI] [PubMed] [Google Scholar]
- Sussman RW, Garber PA, Cheverud JM. Importance of cooperation and affiliation in the evolution of primate sociality. Am J Phys Anthropol. 2005;128:84–97. doi: 10.1002/ajpa.20196. [DOI] [PubMed] [Google Scholar]
- Szameitat DP, Alter K, Szameitat AJ, Wildgruber D, Sterr A, Darwin CJ. Acoustic profiles of distinct emotional expressions in laughter. J Acoust Soc Am. 2009;126:354–366. doi: 10.1121/1.3139899. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hamann K. Collaboration in young children. Q J Exp Psychol. 2012;65:1–12. doi: 10.1080/17470218.2011.608853. [DOI] [PubMed] [Google Scholar]
- van Hooff JARAM. A structural analysis of the social behaviour of a semi-captive group of chimpanzees. In: von Cranach M, Vine I, editors. Expressive movement and non-verbal communication. Academic Press; London: 1973. [Google Scholar]
- Vogel C. Group cohesion, cooperation and synchrony in a social model of language evolution. Development of multimodal interfaces: active listening and synchrony. Lect Notes Comput Sci. 2010;5967:16–32. doi: 10.1007/978-3-642-12397-9_2. [DOI] [Google Scholar]