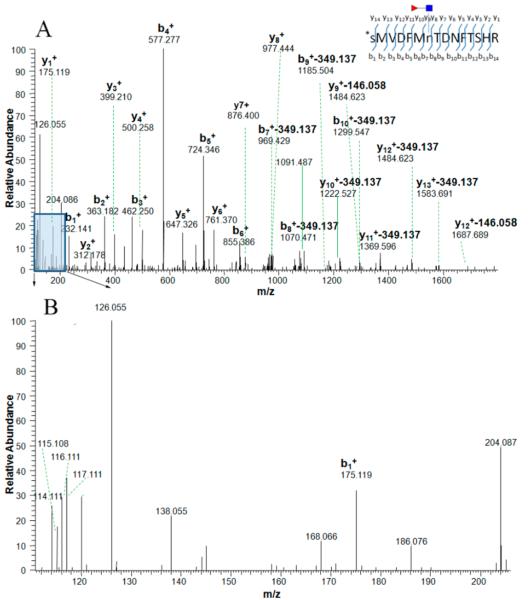
Figure 1.
MS/MS spectra of a tryptic CF peptide, SMVDFMnTDNFTSHR, labeled by iTRAQ (N-terminal amino acid residue) without the N-X-S/T/C consensus motif from protein afamin, where the CF modification was confirmed by diagnostic ions. The ion at m/z 765.763 (2+) was isolated and fragmented by high-energy collision-induced dissociation (HCD) (A). The typical N-linked glycan (NGlcNAc) backbone fragmentation can be identified by the oxonium ions in the lower mass range (B); the signal from the GlcNAc oxonium ion at m/z 126.055 was prominent in the spectrum and oxonium ions at m/z 138.055, 168.088, 186.066, 204.087 were also present. In the higher mass range, the fragment peaks exhibit specific mass differences corresponding to neutral loss product ions and provide information on the CF position on the CF peptide. Product ions with a loss of 349.137 Da (GlcNAcFuc) or 146.058 Da (fucose) are present. Red triangle stands for the fucosyl-GlcNAc glycan residues retained on glycoside.