Abstract
Immune defense is energetically costly, and thus an effective response requires metabolic adaptation of the organism to reallocate energy from storage, growth, and development towards the immune system. We employ the natural infection of Drosophila with a parasitoid wasp to study energy regulation during immune response. To combat the invasion, the host must produce specialized immune cells (lamellocytes) that destroy the parasitoid egg. We show that a significant portion of nutrients are allocated to differentiating lamellocytes when they would otherwise be used for development. This systemic metabolic switch is mediated by extracellular adenosine released from immune cells. The switch is crucial for an effective immune response. Preventing adenosine transport from immune cells or blocking adenosine receptor precludes the metabolic switch and the deceleration of development, dramatically reducing host resistance. Adenosine thus serves as a signal that the “selfish” immune cells send during infection to secure more energy at the expense of other tissues.
A study of the fruit fly's response to parasitoid wasp eggs reveals that immune cells selfishly release adenosine as a signal to trigger a systemic metabolic switch, thereby suppressing nonimmune processes and securing energy and nutrients for immune activity. Read the Primer.
Author Summary
The immune response is energetically costly and often requires adaption of the whole organism to ensure it receives enough energy. It is not well understood how distribution of energy resources within the organism is regulated during an immune response. To understand this better, we used parasitoid wasp infection of fruit fly larvae—the host larvae have 48 h before they pupate to destroy the infecting “alien” or face destruction by the parasitoid that will consume the developing pupa. Here we find a signal, generated by the host immune cells, which mediates a systemic energy switch. This signal—adenosine—suppresses processes driving larval to pupal development of the host, thereby freeing up energy for the immune system. We show that the resulting developmental delay in the fruit fly larvae is crucial for an efficient immune response; without the adenosine signal, resistance to the parasitoid drops drastically. Generation of this signal by immune cells demonstrates that in response to external stressors, the immune system can mobilize reallocation to itself of energy and nutrients from the rest of the organism.
Introduction
Immune response is energetically costly [1,2]. Immune cells, upon activation, favor glycolysis over oxidative phosphorylation for fast, albeit inefficient, energy generation and macromolecule synthesis [3,4]. This metabolic shift requires extra glucose as glycolysis produces much less ATP than does oxidative phosphorylation [5]. Therefore, at the organismal level, the energy shifts from storage and nonimmune processes towards the needs of the immune system [6–9].
Regulation of energy during the immune response is critical—full response requires a significant amount of energy, and inability to provide it with nutrients can lead to immune system suppression and reduced resistance [10–12]. In mammalian systems, the inflammatory cytokines TNF-α, IFN-γ, IL-1, and IL-6 are released upon recognition of the pathogen and, besides modulating immune functions, they also stimulate energy release [2,13–16]. Immune cells must respond rapidly to the activating signals, and thus they change their metabolism, which involves, at least in mammalian systems, the preferential use of aerobic glycolysis, known as the Warburg effect [3,4,17]. The increased demand for energy by the immune system requires, both in vertebrates and invertebrates, adaptation of the whole organism, which is associated with an overall metabolic suppression and a systemic insulin resistance in all tissues except the immune cells [2,12,18]. The importance of the systemic regulation of energy is demonstrated by examples of certain infections leading to depletion of energy reserves (wasting) and eventually death of the organism [15,19]. Despite the importance of the systemic regulation of energy, we have only fragmentary knowledge about the molecular mechanisms involved in the regulation of energy during immune response at the organismal level and about the communication between different parts of the organism mediating the shift of energy from storage and growth towards immunity [12,20,21].
Extracellular adenosine (e-Ado) is a signal originating from damaged or stressed tissues. Acting as an energy sensor, e-Ado is released from metabolically stressed cells with depleted ATP [22,23] or made from extracellular ATP leaking from damaged tissues [24]. e-Ado then works as a local or systemic hormone, adjusting metabolism by acting either via adenosine receptors or by the uptake into the cells and conversion to AMP activating AMP-activated protein kinase (AMPK) [24,25]. These actions lead to a suppression of energy consuming processes [22,26–29] and to a release of energy from stores [30].
Damaged tissues and metabolically stressed cells are very likely to occur during immune response and thus it is not surprising that elevated levels of e-Ado are also detected, for example, during sepsis in humans [31]. The capacity of e-Ado to regulate energy metabolism, to “measure” the level of tissue and organismal stress, and to adapt the energy use to the actual situation all make e-Ado a perfect candidate for an energy regulator during immune response. However, the mode of e-Ado action under immune challenge is unclear, as the role of e-Ado in energy regulation has mainly been studied in relation to anoxia in anoxia-tolerant organisms such as turtles and hypoxia and ischemia in rodent models and human patients [22,30], while e-Ado has thus far been associated with mammalian immune response only through its immunomodulatory and anti-inflammatory function [24,32].
We, and others, have previously shown that adenosine regulatory and signaling network in Drosophila is similar to mammalian systems [33–37]. In addition, we have shown that e-Ado regulates energy metabolism in Drosophila. Increase of e-Ado levels caused by a deficiency of adenosine deaminase-related growth factor A (ADGF-A) leads to hyperglycemia and reduced energy storage [38]. We have also found that the regulation of e-Ado by ADGF-A is particularly important during parasitoid wasp infection in Drosophila larvae; ADGF-A is strongly expressed in immune cells that encapsulate the invading wasp egg [39]. These findings further support a potential role of e-Ado in energy regulation during immune response.
Here, we use the parasitoid wasp infection as a model to study the energy regulation during immune reaction. Parasitoid wasps inject their eggs into Drosophila larvae, and if the fly larva does not destroy the egg in time, the hatched wasp larva will consume the host [40]. The fly larva recognizes the egg and mounts a robust immune response that involves proliferation and differentiation of specialized immune cells, lamellocytes, which eventually encapsulate the parasitoid egg. Using this immune response as a model, we traced the dietary glucose destinations, measured selected metabolites and gene expressions, and analyzed host resistance and the impact of the immune response on its development.
We describe here the systemic changes in energy metabolism during the immune challenge and the role of e-Ado in the regulation of these changes. We have found that e-Ado, released from the immune cells, mediates a metabolic switch characterized by the suppression of nutrient storage and developmental growth in favor of the immune defense. This metabolic switch—a tradeoff between development and defense—is crucial for the resistance to infection. In Drosophila larvae lacking adenosine signaling, development is not suppressed, and the resistance dramatically drops.
Results
Immune Response to Parasitoid Wasp Egg
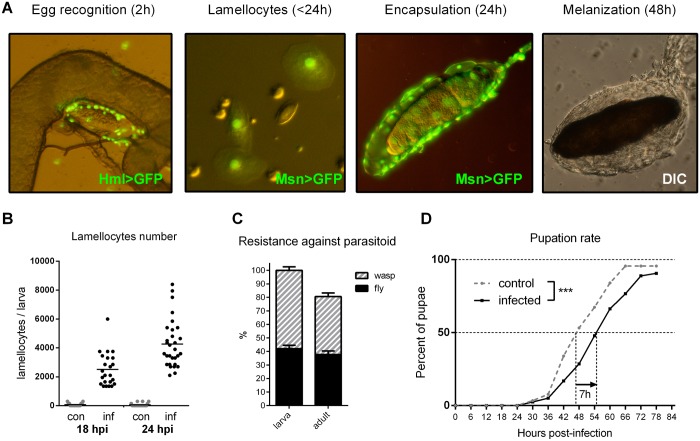
The endoparasitoid wasp Leptopilina boulardi injects its egg in early third-instar Drosophila larva. The egg, usually hiding in gut folds, is first recognized by the host-circulating hemocytes (Fig 1A) and the recognition triggers immune response [40]. This involves production of specialized cells called lamellocytes (Fig 1A and 1B) within the first 24 h postinfection (hpi; 0 hpi is the time of infection and corresponds to 72 h after egg laying; the time in hpi is also used for the uninfected control). Lamellocytes are then released into circulation, and the egg gets encapsulated with subsequent melanization by 48 hpi (Fig 1A). Production of lamellocytes involves a transient proliferation of prohemocytes in the lymph gland and their terminal differentiation into lamellocytes [41]. The efficiency of egg encapsulation depends on the ability to produce lamellocytes and thus varies among different genetic strains of Drosophila [42,43]. Our model was based on the Canton S strain of Drosophila melanogaster bearing the w 1118 mutation (hereafter w), which served as a control genotype in all our experiments (the term “control” is reserved hereafter for uninfected situations, i.e., control w means uninfected w larvae). On average, 42% of these w host larvae succeeded to destroy the wasp egg and 38% survived to adulthood while 42% parasitoids developed to adult wasps (Fig 1C).
Fig 1. Immune response to parasitoid wasp intrusion.
(A) Progressive stages of the response. The wasp egg is recognized by plasmatocytes (green, Hml>GFP) within 2 hpi. Lamellocytes, labeled by the Msn>GFP marker appear in circulation (<24 hpi) and start to encapsulate the egg. At 48 hpi, the egg is fully encapsulated by a multilayer of immune cells and melanized (original image of encapsulation published in [39]). (B) Number of lamellocytes per larva in control (con, grey) and infected (inf, black) larvae at 18 and 24 hpi. Each dot represents an individual larva; the horizontal lines indicate mean. (C) Percentage of host larvae with melanized wasp eggs (black, left column, mean 42%) and surviving host adults (black, right column, mean 38%) against winning wasp larvae and adults (hatched columns). Values are mean ± standard error of measurement (SEM). (D) Pupation of infected larvae (n = 316) was significantly delayed compared to control larvae (n = 344). Log-rank survival analysis (p < 0.0001).
Immune Response to Parasitoid Egg Invasion Demands Energy
Parasitoid-infected third-instar larvae experienced a 15% developmental delay, pupating on average 7 h later than uninfected controls (Fig 1D). Such a delay might result from redistribution of energy from development towards immune defense. We therefore examined various energy aspects during infection.
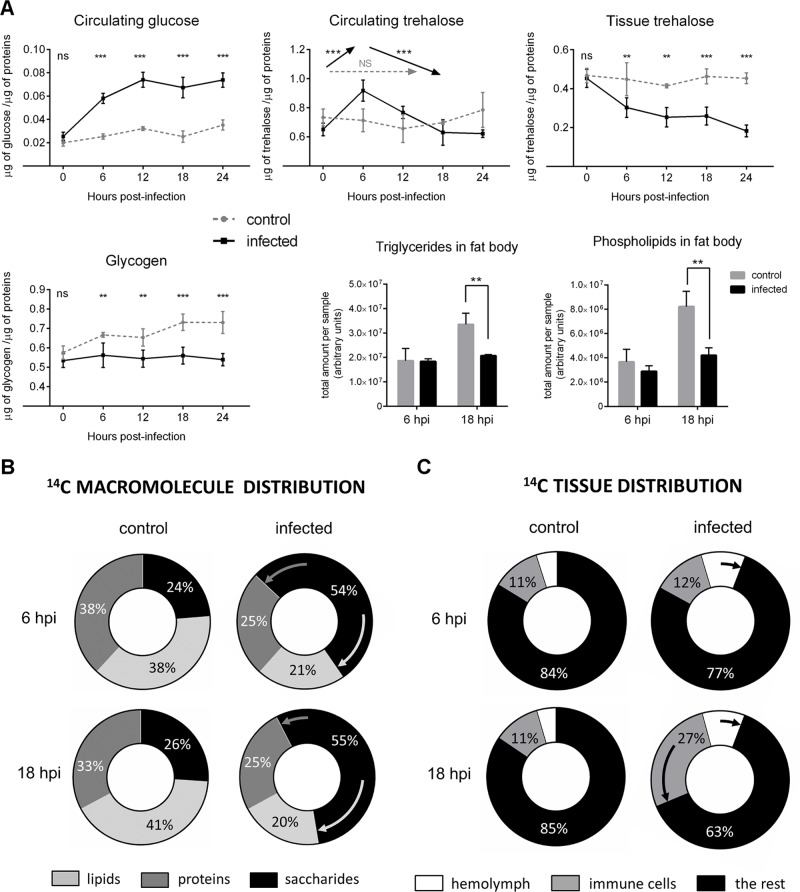
Without infection, circulating glucose was kept below 0.04 μg per μg protein (Fig 2A). Both the glycogen and triacylglycerol (TAG) stores kept increasing, while circulating and tissue trehalose levels remained steady (Fig 2A). Trehalose is a nonreducing disaccharide source of glucose, which is liberated by the action of trehalase [44]. To trace the fate of glucose, we employed dietary radiolabeled D[U-14C]-glucose. The glucose-derived 14C became evenly distributed in the larvae among saccharides, proteins, and lipids (Fig 2B). About 84% of 14C was found in developing tissues (Fig 2C). We divided the organism here in a simplified way into the immune system (represented by cellular immunity, the most important defense against parasitoids, including circulating hemocytes and lymph gland), the circulation (hemolymph), and the rest of the tissues representing mainly development, growth, and energy storage.
Fig 2. Metabolic changes during immune response in w flies.
(A) Nutrient contents in the hemolymph, whole larval lysates, and fat body at different time points after infection (uninfected control: grey dashed line and grey column; infected: solid black line and column). Circulating glucose increases, tissue trehalose decreases, glycogen and lipids accumulation ceases upon infection; circulating trehalose first increases and then decreases making a 6 hpi peak. Values are mean ± SEM of four experiments (three for lipids). Asterisks show statistical significance (*p < 0.05; ** p < 0.005; *** p < 0.0005; ns, not significant) when compared between infected and control samples at indicated time points; arrows (Circulating trehalose, middle) indicate increase, decrease, and no change (NS), respectively, between time points. Significance of differences was tested by two-way ANOVA. (B) Percent incorporation of 14C-labeled dietary glucose into lipids, proteins, and saccharides in whole larvae. Incorporation into lipids and proteins decreases upon infection, enlarging saccharide fraction as indicated by arrows. (C) Percent distribution of 14C into the hemolymph, immune cells (circulating hemocytes, and lymph gland) and the rest of the larvae (brain, imaginal discs, gut, fat body, and carcass). 14C first increases in hemolymph at 6 hpi (from 5% to 10%) and then also in immune cells (from 11% to 27%) at the expense of the rest of the organism upon infection; arrows indicate infection-induced changes. This figure shows data for the w genotype; the same values are shown in subsequent figures when compared with other genotypes. See S2 Fig for statistical analysis.
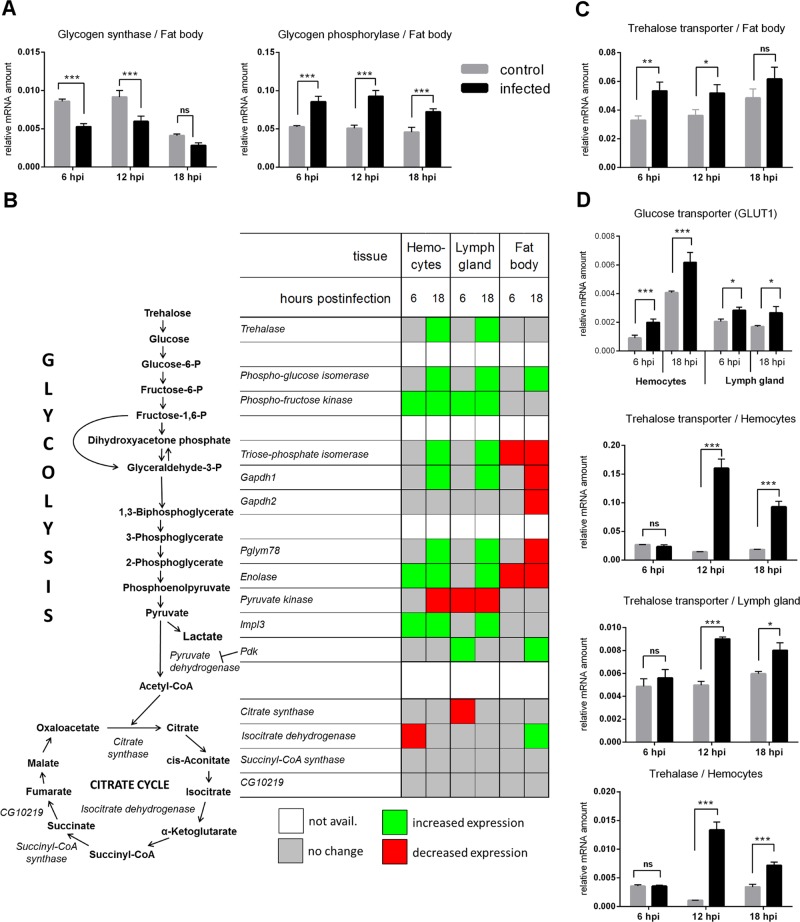
In infected larvae, the accumulation of TAG and glycogen reserves ceased (Fig 2A). This was accompanied by down-regulation of glycogen synthase (CG6904; FlyBase ID: FBgn0266064) and up-regulation of glycogen phosphorylase expression (CG7254; FlyBase ID: FBgn0004507)(Fig 3A). The amount of tissue trehalose decreased (Fig 2A), and less dietary glucose was incorporated into lipids and proteins (Fig 2B and S2 Fig). These hallmarks of suppressed energy storage and growth were corroborated by reduced incorporation of 14C into developing tissues from 84% in uninfected larvae to 77% at 6 hpi and 63% at 18 hpi (Fig 2C and S2 Fig).
Fig 3. Gene expression during immune response of w larvae measured by q-PCR.
(A) Reciprocal changes in mRNA expression of glycogen synthase and glycogen phosphorylase enzymes in the fat body. (B) Summary of significant changes in expression of glycolytic and citrate cycle enzymes in the hemocytes, lymph gland, and fat body (see S3–S5 Figs for corresponding graphs). Heat map indicates a tendency of glycolytic genes to increase in immune cells and to decrease in fat body. (C) Expression of trehalose transporter Tret1-1 in the fat body. (D) Expression of GLUT1, TreT1-1, and trehalase in the circulating hemocytes and lymph gland. All graphs except (B) show mean values of expression relative to Rp49 ± SEM from three independent experiments; grey columns: control larvae, black columns: infected larvae; asterisks indicate significant changes (tested by two-way ANOVA).
The above effects were associated with hyperglycemia as indicated by elevated hemolymph glucose and 14C at the expense of developing tissues (Fig 2A and 2C). Incorporation of 14C into lipids and proteins (at the whole organism level) was also suppressed during infection (Fig 2B), which was accompanied by down-regulation of specific glycolytic enzyme genes in the fat body (Fig 3B and S3 Fig). The diversion of metabolism from building energy reserves and from fat body glycolysis was thus in agreement with extra 14C in the carbohydrate form and with the increase of circulating glucose and trehalose. Circulating trehalose peaked at 6 hpi (Fig 2A) concomitantly with increased expression of a trehalose transporter in the fat body, the organ where trehalose is produced (Fig 3C).
At the same time, the immune cells changed their behavior during infection in the opposite direction, leading to increased energy consumption. Around one-tenth of 14C is normally allocated to immune cells, leaving almost 90% to the rest of the organism, but immune cells demanded up to one-third of nutrients during immune response (Fig 2C). Expression of several glycolytic genes including lactate dehydrogenase Impl3 (CG10160; FlyBase ID: FBgn0001258) increased both in the circulating hemocytes and in the lymph gland (Figs 3B, S4, and S5). This resembled the glucose-demanding aerobic glycolysis, the Warburg effect, in activated mammalian immune cells. Both the lymph gland and the circulating hemocytes expressed elevated amounts of glucose transporter Glut1 (CG43946; FlyBase ID: FBgn0264574) and trehalose transporter Tret1-1 (CG30035; FlyBase ID: FBgn0050035) mRNAs (Fig 3D). Interestingly, later during infection (12–18 hpi), the circulating hemocytes together with already differentiated lamellocytes strongly increased expression of both Tret1-1 and trehalase (CG9364; FlyBase ID: FBgn0003748) (Fig 3D). This suggests that differentiated immune cells preferentially uptake energy in the form of trehalose, which may be linked to the decline of circulating trehalose after 6 hpi (Fig 2A). These results demonstrate a shift of energy distribution away from storage and growth, first towards circulating glucose and trehalose, and then towards the immune cells (Fig 2).
e-Ado Signaling via AdoR Is Required for Hyperglycemia and Effective Immune Response
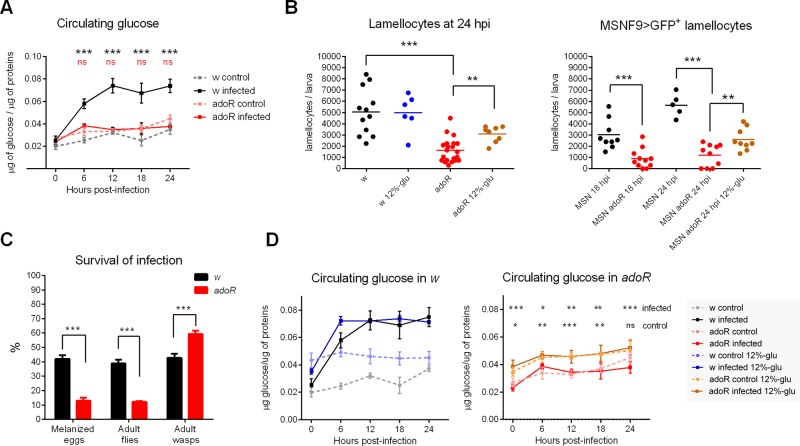
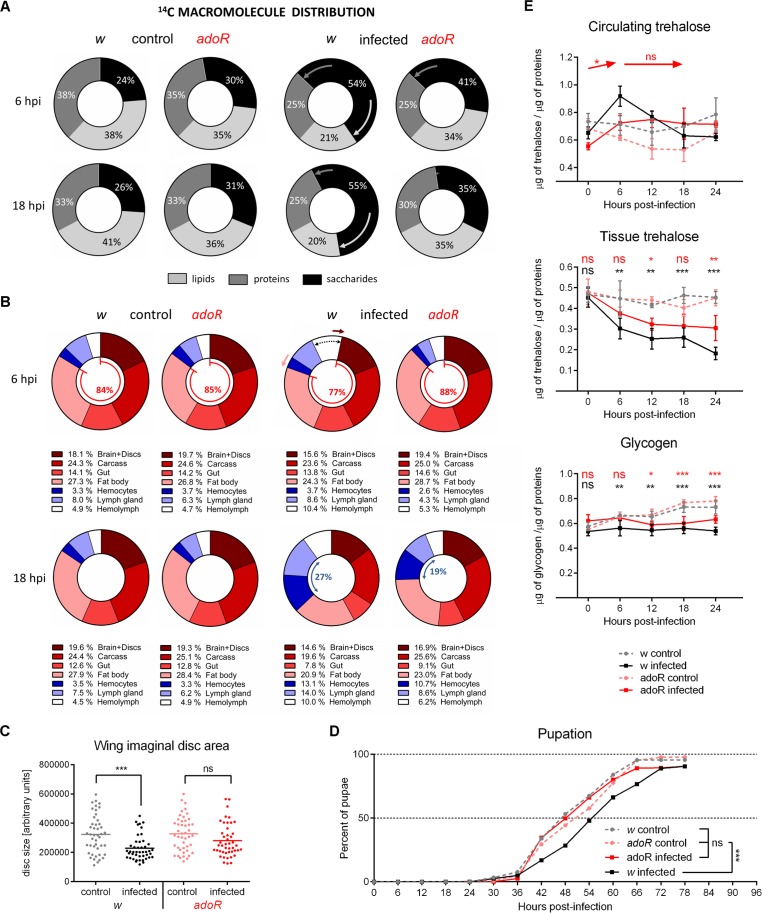
We have previously shown that e-Ado increases circulating glucose via adenosine receptor (AdoR; CG9753; FlyBase ID: FBgn0039747) signaling [38]. Here, we tested if e-Ado was involved in the observed effects of infection on the metabolic shift. While the circulating glucose increased more than 2-fold during infection in w larvae, this increase was suppressed in adoR (FlyBase ID: FBal0191589) mutant larvae (Fig 4A), indicating that AdoR was indeed necessary for the energy redistribution during infection. Therefore, we compared the number of lamellocytes as a measure of immune response. While w larvae produced 5–6 thousand lamellocytes by 24 hpi, the adoR mutants contained less than a third of this amount (Fig 4B). Yet the adoR mutants were clearly capable of differentiating functional lamellocytes that displayed normal morphology, expressed a lamellocyte-specific MSNF9>GFP marker (FlyBase ID:FBtp0064497), and were capable of encapsulating the wasp egg (Fig 4C and S16 Fig). Therefore, adoR larvae were impaired in efficiency or speed of lamellocyte production, and this corresponded with their reduced resistance against the parasitoid invasion relative to w larvae. Indeed, the adoR mutants were three times less successful at neutralizing the wasp eggs and surviving to adult flies (Fig 4C). Thus, AdoR signaling is crucial for effective immune defense against the parasitoid.
Fig 4. Effects of blocking signaling through adoR on immune response.
(A) Increase in circulating glucose level during infection is suppressed in the adoR mutant. Values are mean ± SEM of four experiments; black asterisks—comparison of w; red “ns” (not significant)—comparison of adoR; tested by two-way ANOVA. (B) Number of lamellocytes based on cell morphology and a lamellocyte-specific MSNF9>GFP marker. adoR larvae contain fewer lamellocytes than w or MSN controls. High-glucose diet (12%-glu) increases lamellocyte number in adoR larvae. Each dot represents lamellocyte count per larva, the lines are mean values; tested by unpaired t test. (C) adoR mutation significantly reduces the host resistance to parasitoid wasp as assessed from frequency of melanized eggs (adoR—13% versus w—42%; n = 100 Drosophila larvae per genotype in five experiments), emerged adult flies (adoR—12% versus w—38%; n = 310 for adoR, 316 for w, in three experiments). Values are mean ± SEM; tested by unpaired t test. (D) High-glucose diet (12%-glu) significantly increases circulating glucose both in uninfected w and adoR larvae and in infected adoR larvae (graph with w does not show statistical significance). Values are mean ± SEM of three experiments; tested by two-way ANOVA. In all panels, statistical significance of differences is indicated as *p < 0.05; ** p < 0.005; *** p < 0.0005; and ns, not significant.
The impaired defense in the adoR mutants was not due to affected recognition of the wasp egg, as the number of plasmatocytes attached to the egg surface within the first few hpi was similar in w and adoR larvae (S7 Fig). Therefore, we tested if shortage of energy could be the problem as suggested by failure to increase circulating sugar levels in adoR larvae (Fig 4A). When we fed these larvae a high-glucose diet (12% instead of the regular 5%), the hemolymph glucose significantly increased even without infection in both w and adoR larvae (Fig 4D). This dietary treatment significantly increased the number of lamellocytes in the infected adoR larvae (Fig 4B), suggesting that it was the lack of energy causing inefficient differentiation of lamellocytes in the absence of AdoR.
Interestingly, adding glucose to the diet did not further increase the level of circulating glucose during infection. In fact, the increase induced by infection was greater than that achieved with dietary glucose (Fig 4D), and consistently the number of lamellocytes in infected w larvae was the same on both diets (Fig 4B). Since the glucose increase induced by the dietary treatment was not as high as the one induced by the infection, the number of lamellocytes in adoR did not reach, even on the high-glucose diet, the levels observed in w (Fig 4B). This suggests that the glucose available in circulation is the limiting factor for the lamellocyte differentiation.
AdoR Signaling Mediates the Metabolic Switch
Upon infection, more glucose was retained in the saccharide fraction in the w larvae (Fig 2B), indicating that this glucose was available for energy needs of the immune response and less used for storage and growth. Little (at 6 hpi) or no (18 hpi) such retention was observed in adoR mutants (Fig 5A and S2 Fig), suggesting that storage and/or growth were not suppressed during infection in the absence of AdoR. This notion was supported by the relative distribution of 14C among individual tissues (Fig 5B). The distribution was the same in uninfected w and adoR animals. The incorporation of 14C did not change at 6 hpi in infected adoR (as opposed to w), and the shift from storage and growth (red part) towards immune cells (blue part) was much smaller in infected adoR compared to w at 18 hpi (Fig 5B). Importantly, the comparison of relative distribution of 14C into tissues was allowed by equal total uptake of 14C-glucose from diet in w and adoR larvae (S8 Fig). Interestingly, the comparison of absolute numbers of 14C entering the system also revealed anorexia during infection (lower uptake of 14C; S8 Fig), supporting a common observation during immune responses [45]. This anorexia did not seem to depend on AdoR. Besides the lymph gland with slightly lower 14C in adoR mutants, the tissue distribution of 14C was similar in uninfected w and adoR larvae at both time points (Fig 5B and S10 Fig).
Fig 5. Metabolic changes and developmental effects of AdoR deficiency.
(A) Incorporation of 14C-glucose into lipids and proteins is reduced upon infection in w but not in adoR larvae. Arrows indicate infection-induced changes. For statistical analysis, see S2 Fig. (B) Relative distribution of 14C in the hemolymph (white), immune cells (circulating hemocytes, dark blue; lymph gland, light blue), and the remaining body parts (brain with imaginal discs—brown; carcass, i.e., all the remnants after dissecting all other presented tissues—red; gut—light red; fat body—pink). Arrows indicate increasing 14C in hemolymph (black dashed arrow) of w at 6 hpi at the expense of brain+discs (brown arrow) and fat body (pink arrow); these changes are missing in adoR. Increase in hemolymph and in immune cells (blue arrow) of w at 18 hpi at the expense of all other tissues is smaller in adoR (less in immune cells and more in the rest). Legends below graphs show percentages in body parts. For detailed analysis, see S9 Fig and S10 Fig. (C) Growth of the wing imaginal discs is delayed by infection in w (unpaired t test p < 0.0001) but not in adoR larvae (p = 0.06). Each dot represents measured area of an individual disc at 18 hpi; horizontal lines indicate mean. (D) Pupation is delayed upon infection in w larvae (n = 316, control and 344, infected) but not in adoR larvae (n = 310, control and 293, infected). The rates were compared using Log-rank survival analysis; the p values are: w < 0.0001; adoR = 0.74; w control versus adoR control = 0.053; w control versus adoR infected = 0.054. (E) Nutrient contents in the hemolymph and whole larval lysates. Values are mean ± SEM of four experiments. Circulating trehalose in adoR does not form the 6 hpi peak of w; arrows show increase and no change (ns), respectively, when levels of infected adoR are compared between time points. Tissue trehalose show smaller differences for adoR and glycogen shows similar pattern to w. Asterisks show statistical significance when compared between infected and control animals at indicated time points (black for w, red for adoR). Tested by two-way ANOVA; for statistical analysis, see S2 Fig.
Upon infection, only the brain and imaginal disc complex and fat body of w larvae contained significantly less 14C while hemolymph contained significantly more 14C at 6 hpi (Fig 5B and S9 Fig). While 14C incorporation into brain+discs significantly decreased in w larvae, it did not change in the adoR mutant upon infection (Fig 5B and S9 Fig), demonstrating that the suppression of developmental growth, which occurred during infection, was missing in adoR. This is supported by the measurement of the wing imaginal disc growth. While the growth of discs was significantly delayed in w control upon infection, the delay did not occur in the adoR mutant (Fig 5C). Similarly, the delay in development observed in infected w (as measured by pupation rate) did not occur in adoR, which pupated as there would be no infection (Fig 5D).
At 18 hpi, all tissues were affected by infection, significantly increasing 14C in immune cells and hemolymph and decreasing in the rest (Fig 5B and S9 Fig). In all cases but gut, the changes were significantly smaller in adoR than in w (S10 Fig), indicating that the AdoR signaling was involved in the overall suppression of nonimmune processes.
The missing suppression of development in adoR larvae resulted in shortage of energy available for the immune system as documented first by almost no increase of 14C in the hemolymph at 6 hpi and then by much lower 14C incorporation into the immune cells at 18 hpi compared to infected w larvae (Fig 5B). Weak suppression of nonimmune processes in the absence of AdoR may also be linked to the missing peak of circulating trehalose at 6 hpi (Fig 5E and S2 Fig). Functional AdoR signaling seems to lower glucose transport and to increase trehalose transport in the fat body (suggested by expression levels of the respective transporter genes; S11 Fig), leading to increased trehalose at 6 hpi. The trehalose peak probably serves as a reservoir for fast glucose production, which will be increasingly needed for immune defense. The rapid lamellocyte differentiation is lagging in adoR larvae, likely reflecting lower consumption of trehalose relative to w larvae (Fig 5E).
Effect of Ado Transport on Energy Regulation during Immune Response
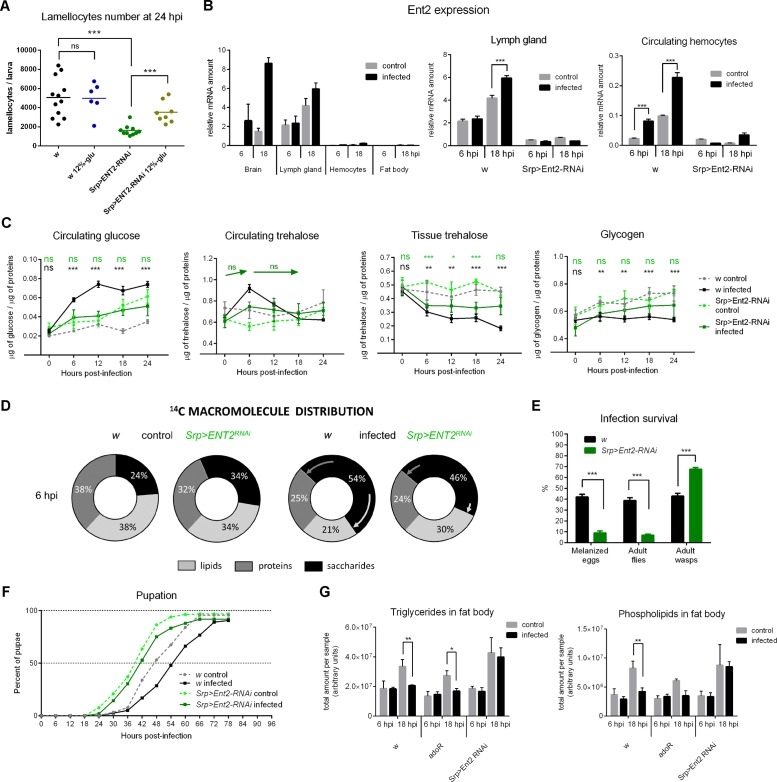
The AdoR signaling reallocates energy towards immune defense, suggesting that e-Ado is released upon immune challenge. Therefore, we next wanted to determine the source of e-Ado during wasp invasion. We individually knocked down the Equilibrative nucleoside transporters, ENT1 (CG11907; FlyBase ID: FBgn0031250) and ENT2 (CG31911; FlyBase ID: FBgn0263916), which are expressed in Drosophila larvae [36,46]. We delivered RNAi to various tissues utilizing the Gal4-UAS system [47], and as a simple readout we used lamellocyte count at 24 hpi (S12 Fig). Among the tested combinations, only ENT2 knockdown driven by Srp-Gal4 (FlyBase ID: FBtp0020112) in cells of the hematopoietic lineage achieved a reduction in the number of lamellocytes that resembled the effect of adoR deficiency (Figs 4B, 6A, and S12). Srp-Gal4 was expressed in all hematopoietic cells, including the circulating hemocytes and all cells of the lymph gland that also contained precursors of lamellocytes (S13 Fig). In contrast, knocking down ENT2 in already differentiated hemocytes (by Hml-Gal4 and Upd3-Gal4 drivers; FlyBase ID: FBtp0040877 and FBtp0020110) did not affect the lamellocyte number (S12 Fig).
Fig 6. Effects of blocking adenosine transport in immune cells by ENT2 RNAi.
(A) Srp>ENT2-RNAi reduces lamellocyte number compared to w larvae, and high dietary glucose (12%-glu) significantly increases the lamellocyte number in Srp>ENT2-RNAi. Each dot represents lamellocyte count per larva; lines indicate mean. Differences were tested by unpaired t test. (B) ENT2 mRNA expression. Comparison of ENT2 mRNA expression in various tissues in w larvae shows a strong expression in brain and lymph gland, increasing in both upon infection. Srp>ENT2-RNAi reduces the ENT2 expression below 20% both in the lymph gland and hemocytes. Values are mean ± SEM of relative expression (normalized to Rp49 mRNA) of three experiments; tested by two-way ANOVA. (C) Nutrient contents in the hemolymph and whole larval lysates. Circulating glucose does not increase in Srp>ENT2-RNAi upon infection. Circulating trehalose in Srp>ENT2-RNAi does not form the 6 hpi peak of w; arrows indicate no change (ns) in levels of infected Srp>ENT2-RNAi when compared between time points. Tissue trehalose shows similar pattern to w. Glycogen does not differ between control and infected Srp>ENT2-RNAi indicating an accumulation of stores even upon infection. Asterisks show statistical significance when compared between infected and control animals at indicated time points (black for w, green for Srp>ENT2-RNAi). Values are mean ± SEM of four experiments; tested by two-way ANOVA. For statistical analysis, see S2 Fig. (D) Incorporation of 14C-glucose into lipids and proteins is reduced upon infection in w larvae but significantly less so in Srp>ENT2-RNAi larvae. Arrows indicate infection-induced changes. For statistical analysis, see S14 Fig. (E) Srp>ENT2-RNAi significantly reduces the host resistance to parasitoid wasp as assessed from frequency of melanized eggs (9% versus 42%; n = 100 Drosophila larvae per genotype in five experiments) and emerged adult flies (7% versus 38%; n = 316 for w, 343 for Srp>ENT2-RNAi in three experiments). Values are mean ± SEM; tested by unpaired t test. (F) Uninfected Srp>ENT2-RNAi larvae (n = 377) pupate 8 h earlier than uninfected w larvae, and infection only delays their pupation by 2 h (n = 343). Compared using Log-rank survival analysis (p < 0.0001 for all comparisons). (G) Total amount of TAG and phospholipids in the fat body of w, adoR, and Srp>ENT2-RNAi larvae. While infection suppresses TAG storage in w and adoR, TAG grows unaffected by infection in Srp>ENT2-RNAi. Data are mean values of mass spectra peak area per sample ± SEM; tested by two-way ANOVA.
ENT2 mRNA was abundant in the lymph gland and brain but weakly expressed in circulating hemocytes and virtually undetected in the fat body (Fig 6B). During infection, ENT2 expression increased in all these tissues except the fat body (Fig 6B) and, consistently, ENT2 RNAi delivered using a fat body-specific C7-Gal4 driver did not affect the number of lamellocytes (S12 Fig). The increasing expression of ENT2 during infection in the brain leaves a possibility that the nervous system contributes e-Ado; however, undetectable expression of Srp-Gal4 in the brain, except for minor signal in some nerve cords (S13 Fig), makes the observed effects of ENT2 removal attributable to the immune cells.
The results above suggest that Ado transport from immune cells, including the differentiating ones, is important for efficient lamellocyte differentiation. As in the case of adoR mutation (Fig 4B), the loss of lamellocytes was rescued by increasing dietary glucose in the Srp>ENT2-RNAi larvae (Fig 6A). Similarly to adoR mutation, ENT2 knockdown in immune cells also cancelled changes in nutrient distribution that normally take place in infected w larvae; there was no peak of circulating trehalose at 6 hpi and no increase in circulating glucose (Fig 6C and S2 Fig). The partition of 14C into saccharides, proteins, and lipids also resembled the pattern seen in adoR mutant larvae (compare Fig 6D with Fig 5A and S2 Fig with S14 Fig).
Together, the above data indicate that deficiency in e-Ado release and in its receptor, AdoR, consistently lead to the same failure of energy reallocation during immune challenge. Indeed, like loss of AdoR, knocking down ENT2 also reduced the host resistance against wasp invasion (Fig 6E), while the normal developmental delay observed in w controls upon infection did not occur in Srp>ENT2-RNAi larvae (Fig 6F). Interestingly, pupation occurred earlier in Srp>ENT2-RNAi compared to w or adoR animals even without infection (Fig 6F); the size of pupae was unaffected implying faster growth instead of precocious pupation of Srp>ENT2-RNAi.
While glycogen storage was suppressed similarly upon infection in adoR mutant and w larvae (Fig 5E), there was no significant difference in glycogen content between infected and uninfected Srp>ENT2-RNAi larvae (Fig 6C). Even more apparent was the effect on lipid storage where the accumulation of TAG in the fat body was suppressed both in w and adoR but not at all in Srp>ENT2-RNAi larvae (Fig 6G). Blocking Ado transport thus led to continued nutrient storage even upon immune challenge, suggesting that energy storage during infection might be regulated by e-Ado independently of AdoR.
Discussion
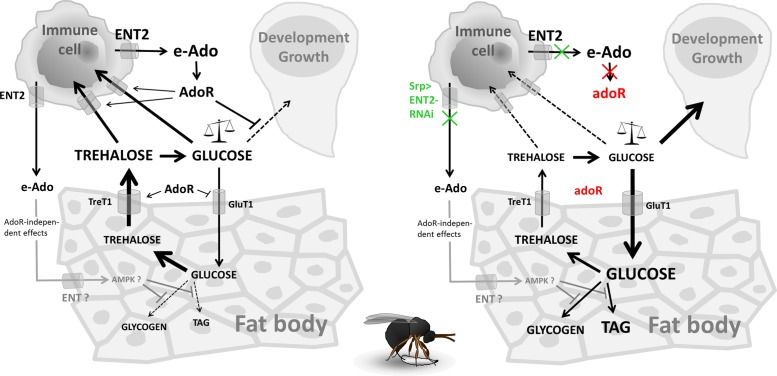
An overall metabolic suppression is a common host response to infection [18,12,6]. A likely purpose for the suppression is to conserve energy for the immune response that is energetically costly [2,12]. The defense of the Drosophila larva against the parasitoid wasp requires a rapid production of specialized immune cells (lamellocytes) that encapsulate the parasitoid egg. This has provided us with a unique in vivo model to study the metabolic changes and their regulation during immune response. We show here that the production of lamellocytes is an energetically demanding process, and that a systemic metabolic switch is required for their effective differentiation. This switch includes (1) suppression of energy storage and developmental growth, (2) retaining more energy in circulation, and (3) increased consumption of energy by the immune system (Fig 7).
Fig 7. Model of metabolic shifts mediated by e-Ado during immune response.
Left—wild-type situation upon infection. Right—situation without e-Ado upon infection; blocking AdoR signaling by adoR mutation is marked in red, blocking Ado transport from immune cells by Srp>ENT2-RNAi is marked in green. See text for details.
Suppression of energy storage (glycogen and lipids) and suppression of growth, as documented by slower growth of imaginal discs, lead to a developmental delay. We show here that e-Ado is a signal mediating this metabolic switch. Blocking this signal then demonstrates that the metabolic switch is crucial for an effective immune response. Without this signal, development and growth proceed at a normal speed, thus reducing energy available to the immune cells. Insufficiency of immune cells due to the shortage of energy then leads to a drastically reduced resistance against the parasitoid. Experimental interference with e-Ado or its receptor, AdoR, thus demonstrates the importance of tradeoff between development and immune response, and identifies e-Ado as a signal responsible for the switch.
Blocking Ado transport from immune cells by knocking down the equilibrative nucleoside transporter ENT2 identified the differentiating immune cells as an important source of the signal for the metabolic switch. This suggests that the immune cells could autonomously regulate energy influx based on their acute needs. Ado is a fine sensor of the cellular energy state, as it becomes produced when the ATP:AMP ratio decreases [23]. This scenario is appealing mainly because immune cells dramatically change their metabolism upon activation, leading to increased aerobic glycolysis akin to the Warburg effect [3,4]. Our expression analysis of glycolytic genes, glucose and trehalose transporters, and 14C uptake by immune cells suggested a similar behavior for the differentiating immune cells upon wasp attack. The ability to rapidly react to a metabolic stress could be why ENT2 is strongly expressed in the lymph gland and the brain, both privileged organs from the energy point of view.
AdoR signaling is important for the suppression of developmental growth. Normally, infection leads to lower consumption of energy by the brain and imaginal discs (later also by other tissues), but the consumption continues in adoR-deficient larvae as if they were uninfected. At the same time, AdoR signaling seems to lower glucose transport and to increase trehalose transport in the fat body as inferred from expression levels of the respective transporter genes. The fat body is the site where trehalose is produced from glucose [44]; trehalose is then released back to the hemolymph, and more so during infection. The adoR mutation causes a misbalance of glucose and trehalose transport in the fat body, causing more nutrients to be retained there. The effect of AdoR signaling on the fat body combined with the suppression of developmental growth leads to hyperglycemia that in turn ensures enough energy to supply the immune cells. If the growth suppression fails to occur, as in the adoR mutant, the immune cells are unable to compete with developing tissues that consume the majority of energy. By analogy to the selfish brain theory [48], “selfish” immune cells may usurp energy to themselves by way of AdoR-mediated silencing of nonimmune processes. Our work thus brings experimental evidence and explains the molecular mechanism for recently published theoretical concept of selfish immune system [49].
Interestingly, the AdoR signaling does not mediate the suppression of energy storage (glycogen and TAG) during infection. However, increasing glycogen and TAG stores in infected Srp>ENT2-RNAi larvae with blocked Ado transport from immune cells indicates that the storage suppression is also under e-Ado control but through an AdoR-independent mechanism. Such a mechanism, which needs to be further studied, may involve e-Ado uptake, conversion to AMP by adenosine kinase, and activation of AMPK [25]. The Srp>ENT2-RNAi larvae proceeded faster through development not only during infection but even without infection when compared to control larvae. This suggests that the regulation of energy storage by e-Ado may play a role even during normal development.
e-Ado signaling was previously associated with regulation of hemocyte differentiation, and blocking the AdoR signaling was suggested to lower the differentiation in the lymph gland under noninfectious conditions [50]. The hallmark of lamellocyte differentiation upon parasitoid wasp infection is the turning off the Jak-Stat signaling in the medullary zone of the lymph gland containing the prohemocytes [51]. Expression of cytokine Upd3 (CG33542; FlyBase ID: FBgn0053542) is down-regulated, and the ratio of Jak-Stat receptor Domeless (CG14226; FlyBase ID: FBgn0043903) and its negative coreceptor Latran (CG14225; FlyBase ID: FBgn0031055) is switched upon wasp infection leading to turning off the Jak-Stat and to induction of lamellocyte differentiation [52]. The expression patterns of Upd3, Domeless and Latran mRNAs normally and during infection are unaffected both in adoR and Srp>ENT2-RNAi (S15 Fig), indicating that the induction of lamellocyte differentiation is functional in these lines. In addition, the lymph glands develop normally in both adoR and Srp>ENT2-RNAi ([50] and S17 Fig). Our results demonstrate that the adoR and Srp>ENT2-RNAi larvae are capable of lamellocyte differentiation; they are just less effective, and the reason is most likely the lack of energy as indicated by the rescue of this phenotype with extra dietary glucose.
An important part of the global energy switch observed upon parasitoid invasion is the AdoR-mediated suppression of developmental growth. Although AdoR is relatively strongly expressed in imaginal discs [34], we do not know if it is the tissue-autonomous signaling of AdoR, or whether AdoR acts systemically on metabolism as AdoR is also strongly expressed in the larval endocrine glands and brain; both scenarios may apply simultaneously. It is known that the activation of adenosine receptor leads to metabolic suppression—at the individual cell level, the activation can inhibit growth of tumor cells [26], but it can also cause a systemic suppression during anoxia [28,29] or torpor [27]. Our work demonstrates that the AdoR-mediated suppression plays an important role also during immune response. It will be important to identify the target cells and signaling cascades mediating the observed suppression in future studies.
We show here that the metabolic switch is mediated by e-Ado and that the switch is crucial for an effective immune response. It is of interest to see if this e-Ado role is common to other organisms including humans. e-Ado plays the same role in energy regulation in flies and mammalian systems [30,38]. For example, sepsis is associated with hyperglycemia and insulin resistance as well as with increased e-Ado [31,53], suggesting that e-Ado could indeed mediate the systemic metabolic switch in higher organisms. However, analyzing this role of e-Ado in mammals will be complicated by the existence of multiple adenosine receptors with partly contradicting functions [54,55] and by diverse roles of e-Ado in immunomodulation [24,56,57].
In conclusion, our study demonstrates that extracellular adenosine, released from immune cells, mediates a systemic metabolic switch leading to suppression of energy storage and developmental growth, thus leaving more energy to the immune cells. This switch is crucial for the effective immune response and blocking adenosine signaling drastically reduces host resistance to the pathogen. This may resemble a selfish brain theory in a way that the immune system, like the brain, is a privileged part of the organism, capable of suppressing energy consumption by other tissues in its own interest. Such a selfish immune system [49] would use e-Ado as a signal to appropriate extra energy resources during immune challenge.
Materials and Methods
Fly Stocks, Culture, and Infection
All strains were backcrossed at least ten times to w 1118 genetic background; w 1118 was used as a control in all experiments. adoR mutant was homozygous for adoR 1 mutation (FBal0191589). RNAi lines originated from VDRC: UAS-Ent1-RNAi (ID 109885) and UAS-Ent2-RNAi (ID 100464). SrpD-Gal4, Upd3-Gal4, and MSNF9-GFP were obtained from Michele Crozatier, HmlΔ-Gal4 from Bruno Lemaitre and C7-Gal4 from Marek Jindra. Flies were grown on cornmeal medium (8% cornmeal, 5% glucose, 4% yeast, 1% agar) at 25°C. For dietary treatment, larvae were transferred upon infection to cornmeal diet with 12% instead of 5% glucose. Early 3rd instar larvae were infected by parasitoid wasp L. boulardi. Weak infection (1–2 eggs per larva) was used for resistance and pupation analysis; strong infection (4–7 eggs per larva) was used in all other cases.
Resistance and Pupation
To determine pupation rate and resistance to parasitoids, infected and control larvae were placed into fresh vials (1 experiment = 30 larvae per vial, 3 vials per genotype; 4 independent experiments). Pupation rate was determined by counting newly appeared pupae every 6 h and incremental percentage of number of pupae per total number of infected and control larvae at a particular time point postinfection was plotted; Log-rank survival analysis was used for comparison. For resistance, we first dissected 20 larvae per experiment from each genotype to count fully melanized wasp eggs (winning host) or surviving wasp larvae (winning parasitoid). Second, we counted all emerged adult flies as surviving the infection and flies without any egg (i.e., uninfected individuals) were excluded from the total number in the experiment. Adult wasps emerged from the vial were counted as adult parasitoid winners.
Gene Expression
Expression was analyzed by quantitative real-time PCR. Samples were collected from three independent infection experiments with three technical replicates for each experiment. Expression was normalized to Ribosomal protein Rp49.
14C-glucose Distribution
Larvae were fed either 73 h AEL or 91 h AEL for 20 min a diet containing D[U-14C]-glucose (10.6 Gbq/mmol; Amersham Biosciences) in yeast. Samples were collected 5 h later. Each sample contained tissues from 30 larvae—all hemolymph was collected by ripping larvae in PBS, centrifuging them, and dividing them into pelleted hemocytes and hemolymph fractions; brains with attached discs and wing discs, whole guts, whole fat bodies, and lymph glands were separated by dissection, and the rest were used as carcass. Macromolecular fractions were separated from tissue homogenates according to [58] for saccharides and lipids and by TCA treatment for proteins. Part of the homogenate was used for measurement of total absorbed amount of 14C molecules. Number of 14C disintegrations per minute was detected by liquid scintillator.
Metabolites Measurement
Glucose, trehalose, and glycogen were measured as described [59], using GAGO-20 kit (Sigma). Lipids extracted with chlorophorm:methanol were quantified by HPLC and mass spectrometry.
Imaginal Disc Size Measurement
Wing discs were dissected from larvae at 90 h AEL (18 hpi), and their size was determined from micrographs by FIJI software.
Data Analysis
Data were analyzed by GraphPad Prism 6 (GraphPad Software, Inc.).
Extended Materials and Methods are available in S1 Text.
Supporting Information
Fig 1 —data.pzfx: Immune response to parasitoid wasp intrusion. Fig 2 —data.pzfx: Metabolic changes during immune response in w flies. Fig 3 —data.pzfx: Gene expression during immune response of w larvae measured by q-PCR. Fig 4 —data.pzfx: Effects of blocking signaling through adenosine receptor (adoR) on immune response. Fig 5 —data.pzfx: Metabolic changes and developmental effects of AdoR deficiency. Fig 6 —data.pzfx: Effects of blocking adenosine transport in immune cells by ENT2 RNAi. S2 Fig—data.pzfx: Statistical analysis of metabolite changes. S3 Fig—data.pzfx: Gene expression analysis of glycolytic and citrate cycle genes in fat body by q-PCR. S4 Fig—data.pzfx: Gene expression analysis of glycolytic and citrate cycle genes in circulating hemocytes by q-PCR. S5 Fig—data.pzfx: Gene expression analysis of glycolytic and citrate cycle genes in lymph gland by q-PCR. S7 Fig—data.pzfx: Recognition of parasitoid wasp egg by plasmatocytes is not affected by adoR. S8 Fig—data.pzfx: Total absorption of 14C from food. S9–S10 Fig—data.pzfx: Comparison of relative 14C-tissue distribution between infected and uninfected larvae and between w and adoR. S11 Fig—data.pzfx: q-PCR expression analysis of genes involved in transport and metabolism of glucose and trehalose in w and adoR. S12 Fig—data.pzfx: Number of lamellocytes in flies with knocked-down equilibrative nucleoside transporters ENT1 and ENT2 in different tissues. S14 Fig—data.pzfx: Relative 14C incorporation into macromolecules in w and Srp>ENT2-RNAi at 6 hpi. S15 Fig—data.pzfx: Expression analysis of genes involved in regulation of lamellocytes differentiation by q-PCR.
(ZIP)
Top timescale—sampling and treatment description for experiments with 14C-labeled glucose. Middle timescale—sample collection and treatment for experiments characterizing reaction to infection. Bottom timescale—sample collection for experiments characterizing metabolites.
(TIF)
A) Statistical analysis of infection-induced changes in circulating trehalose between infection and control in particular time points (upper table) and between different time points either in control or infection (lower table); tested by two-way ANOVA, B) comparison of incorporation of 14C into macromolecules (saccharides, lipids, and proteins), and C) into three distinguished processes (development and growth, cellular immunity, and circulation) in w and adoR; tested by two-way ANOVA. Uninfected individuals marked as CON (grey columns), infected individuals marked as INF (black columns). Graphs show mean values ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
Infection-induced difference in gene expression was analyzed in w and adoR, 6 and 18 hpi. Uninfected individuals are represented by grey columns (CON), infected individuals by black columns (INF). Graphs show mean values relative to Rp49 ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005); tested by one-way ANOVA. Gene symbols and the corresponding genes can be found in S1 Table.
(TIF)
Infection-induced difference in gene expression was analyzed in w and adoR, 6 and 18 hpi. Uninfected individuals are represented by grey columns (CON), infected individuals by black columns (INF). Graphs show mean values relative to Rp49 ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005); tested by one-way ANOVA. Gene symbols and the corresponding genes can be found in S1 Table.
(TIF)
Infection-induced difference in gene expression was analyzed in w and adoR, 6 and 18 hpi. Uninfected individuals are represented by grey columns (CON), infected individuals by black columns (INF). Graphs show mean values relative to Rp49 ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005); tested by one-way ANOVA. Gene symbols and the corresponding genes can be found in S1 Table.
(TIF)
Comparison of infection-induced differences in gene expression between w and adoR in three different tissues (hemocytes, lymph gland, and fat body) and two different time points postinfection (6 and 18 hpi). Green squares—increased expression, red squares—decreased expression, grey squares—no significant difference, white squares—not analyzed. Level of significance p < 0.05; one-way ANOVA.
(TIF)
(A) Percentage of eggs with certain number of plasmatocytes (none, 1–10, 10–20, or >20) attached to their surface within the first 2 hpi and 4–6 hpi in w and adoR mutant larvae. (B) Examples of attached Hml>GFP-labeled hemocytes (green fluorescence) to parasitoid wasp egg within the first 2 hpi and 4–6 hpi in w and adoR mutant larvae.
(TIF)
Larvae were fed 14C-glucose (in blue dye-labeled diet) for 20 min and then transferred to normal diet for 5 h in which they absorbed 14C-glucose and cleared their guts. They were then homogenized to analyze how much 14C-glucose they absorbed. There is no difference in absorption between control w and control adoR or infected w and infected adoR both at 6 and 18 hpi (labeled NS for not significant). Interestingly, infected larvae (both w and adoR) absorbed less 14C than control larvae indicating anorexia upon infection. Graph shows uninfected w (grey columns), infected w (black columns), uninfected adoR (pink columns), and infected adoR (red columns) mean values of disintegration of 14C per minute (dpm) per sample ± SEM of three independent experiments, tested by one-way ANOVA. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
This figure serves as an alternative for Fig 5B to visualize statistical significance. Compared values: uninfected w (grey columns) with infected w (black columns) and uninfected adoR (pink columns) with infected adoR (red columns). Graph shows mean values ± SEM of three independent experiments. Tested by one-way ANOVA with Arc-Sin transformation. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
This figure serves as an alternative for Fig 5B to visualize statistical significance. Compared values: uninfected w (grey columns) with uninfected adoR (pink columns) and infected w (black columns) with infected adoR (red columns). Graph shows mean values ± SEM of three independent experiments. Tested by one-way ANOVA with Arc-Sin transformation. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
Graphs display infection-induced differences in expression level of Trehalose transporter TreT1-1, Glucose transporter 1 (Glut1), Glycogen synthase (Gsyn), and Glycogen phosphorylase (Gps) in fat body, lymph gland, and circulating hemocytes at 6 and 18 hpi. Uninfected individuals marked as CON (grey columns), infected individuals marked as INF (black columns). Graph shows mean values ±SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005; ns for nonsignificant difference); tested by one-way ANOVA.
(TIF)
RNAi was induced by driving UAS-Ent1-RNAi (VDRC ID 109885) and UAS-Ent2-RNAi (VDRC ID 100464) by various Gal4 drivers: Srp-Gal4 expressed in all cells of hematopoietic lineage and fat body, Upd3-Gal4 and Hml-Gal4 in differentiated hemocytes and C7-Gal4 in fat body. Lamellocytes were counted based on morphology using DIC at 24 hpi. Only combination of Srp>Ent2-RNAi significantly decreased lamellocytes. Results were tested by one-way ANOVA, each point in graph represents number of lamellocytes in one individual larva. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005; NS for nonsignificant difference).
(TIF)
Srp-Gal4 driver expression was visualized by crossing to UAS-GFP. Strong expression was detected in all cells of hematopoietic lineage as demonstrated by the GFP fluorescence in circulating hemocytes and all cells in the lymph gland. The expression was also detected in fat body but it was undetectable in the brain besides weak expression in nerve cords. Left panels show DIC image corresponding to GFP fluorescence images on right.
(TIF)
Comparison of incorporation of 14C into macromolecules (saccharides, lipids, and proteins) tested by two-way ANOVA. Uninfected individuals are marked as control (grey columns), infected individuals are marked as infected (black columns). Graphs show mean values ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005; ns for not significant).
(TIF)
RNA was isolated from dissected lymph glands at 6 hpi of control (grey) and infected (black) larvae. Graphs show the reciprocal changes in expression of Jak-Stat receptor Domeless and its negative coreceptor Latran and turning off Unpaired3 (Upd3) upon infection. This hallmark of induction of lamellocyte differentiation does not differ in adoR or Srp>ENT2-RNAi compared to w. Graph shows mean values ± SEM of three independent experiments. Differences were tested by one-way ANOVA; results shown below the graphs—ns = not significant; *<0.05; **<0.005; ***<0.0005; ****<0.00005).
(TIF)
(A) Expression of MSNF9>GFP (green) together with plasmatocytes-specific P1 marker (red) in the lymph gland upon infection at 12 and 18 hpi. Both w and adoR express the lamellocyte marker indicating that adoR is able to differentiate lamellocytes but there is usually less MSNF9>GFP signal in adoR at 12 hpi. While w sometimes already releases lamellocytes into circulation at 18 hpi (as demonstrated by disintegrated lymph gland in DIC picture), adoR has lymph gland still compact at 18 hpi, but with increasing number of MSNF9>GFP positive cells further demonstrating ability of adoR to differentiate lamellocytes but with lower speed. Top—DIC, bottom—fluorescence confocal image. (B) MSNF9>GFP positive lamellocytes in circulation at 27 hpi are present in both w and adoR and their morphology is indistinguishable. Fluorescence and DIC-combined micrographs.
(TIF)
Morphology and zonation of the lymph gland in 72-h-old larvae (corresponding to time of infection) was checked by expression of medullary zone-specific Dome>GFP marker (prohemocyte containing zone; green) and differentiated plasmatocyte-specific P1 marker for cortical zone (red) in w and adoR. Only P1 marker was used in Srp>ENT2-RNAi and DAPI (nuclear staining) for overall morphology to define medullary zone by the absence of P1. In all three genotypes, the zonation and morphology was comparable for multiple samples indicating that there is no gross effect of the used genetic manipulations on the lymph gland development prior to infection.
(TIF)
(XLSX)
(DOCX)
Acknowledgments
We thank the Vienna Drosophila RNAi Center, Michele Crozatier for flies, wasps, and antibodies and Bruno Lemaitre for flies. We thank Marek Jindra for help with writing the manuscript and David Schneider and members of Schneider laboratory for inputs and discussions.
Abbreviations
- ADGF-A
adenosine deaminase-related growth factor A
- AdoR
adenosine receptor
- AMPK
AMP-activated protein kinase
- e-Ado
extracellular adenosine
- hpi
hours postinfection
- SEM
standard error of measurement
- TAG
triacylglycerol
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Grant Agency of the Czech Republic (Project P305-12-0115; www.gacr.cz) and Marie Curie International Outgoing Fellowship within the EU Seventh Framework Programme for Research and Technological Development 2007-2013 (Project 298186; http://ec.europa.eu/research/mariecurieactions/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fong YM, Marano M a, Moldawer LL, Wei H, Calvano SE, Kenney JS, et al. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990;85: 1896–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267: 543–560. 10.1111/j.1365-2796.2010.02218.x [DOI] [PubMed] [Google Scholar]
- 3. Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345: 1250684 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delmastro-Greenwood MM, Piganelli JD. Changing the energy of an immune response. Am J Clin Exp Immunol. 2013;2: 30–54. [PMC free article] [PubMed] [Google Scholar]
- 5. Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292: 504–507. [DOI] [PubMed] [Google Scholar]
- 6. Clark RI, Tan SWS, Péan CB, Roostalu U, Vivancos V, Bronda K, et al. MEF2 Is an In Vivo Immune-Metabolic Switch. Cell. 2013;155: 435–447. 10.1016/j.cell.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, Clay TM, et al. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J Virol. 2007;81: 1796–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rynes J, Donohoe CD, Frommolt P, Brodesser S, Jindra M, Uhlirova M. Activating Transcription Factor 3 Regulates Immune and Metabolic Homeostasis. Mol Cell Biol. 2012;32: 3949–3962. 10.1128/MCB.00429-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoo J-Y, Desiderio S. Innate and acquired immunity intersect in a global view of the acute-phase response. Proc Natl Acad Sci U S A. 2003;100: 1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calder PC. Feeding the immune system. Proc Nutr Soc. 2013;72: 299–309. 10.1017/S0029665113001286 [DOI] [PubMed] [Google Scholar]
- 11. Matarese G, La Cava A, Sanna V, Lord GM, Lechler RI, Fontana S, et al. Balancing susceptibility to infection and autoimmunity: a role for leptin? Trends Immunol. 2002;23: 182–187. [DOI] [PubMed] [Google Scholar]
- 12. Rauw WM. Immune response from a resource allocation perspective. Front Genet. 2012;3: 267–267. 10.3389/fgene.2012.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arsenijevic D, Garcia I, Vesin C, Vesin D, Arsenijevic Y, Seydoux J, et al. Differential roles of tumor necrosis factor-a and interferon-g in mouse hypermetabolic and anorectic responses induced by LPS. Eur Cytokine Netw. 2000;11: 662–668. [PubMed] [Google Scholar]
- 14. Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25: 193–200. [DOI] [PubMed] [Google Scholar]
- 15. Tracey KJ, Cerami A. Tumor Necrosis Factor and Regulation of Metabolism in Infection: Role of Systemic versus Tissue Levels. Exp Biol Med. 1992;200: 233–239. [DOI] [PubMed] [Google Scholar]
- 16. Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. 1997;82: 4167–4170. [DOI] [PubMed] [Google Scholar]
- 17. Wolowczuk I, Verwaerde C, Viltart O, Delanoye A, Delacre M, Pot B, et al. Feeding our immune system: impact on metabolism. Clin Dev Immunol. 2008;2008: 639803 10.1155/2008/639803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chambers MC, Song KH, Schneider DS. Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster. PLoS ONE. 2012;7: e50679 10.1371/journal.pone.0050679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol. 2006;16: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 20. Dionne M. Immune-metabolic interaction in Drosophila. Fly (Austin). 2014;8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hull-Thompson J, Muffat J, Sanchez D, Walker DW, Benzer S, Ganfornina MD, et al. Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz. PLoS Genet. 2009;5: e1000460 10.1371/journal.pgen.1000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buck LT. Adenosine as a signal for ion channel arrest in anoxia-tolerant organisms. Comp Biochem Physiol Part B. 2004;139: 401–414. [DOI] [PubMed] [Google Scholar]
- 23. Newby AC. Adenosine and the concept of “retaliatory metabolites.” Trends Biochem Sci. 1984;9: 42–44. [Google Scholar]
- 24. Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112: 358–404. [DOI] [PubMed] [Google Scholar]
- 25. Da Silva CG, Jarzyna R, Specht A, Kaczmarek E. Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res. 2006;98: e39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fishman P, Bar-Yehuda S, Barer F, Madi L, Multani AS, Pathak S. The A3 Adenosine Receptor as a New Target for Cancer Therapy and Chemoprotection. Exp Cell Res. 2001;269: 230–236. [DOI] [PubMed] [Google Scholar]
- 27. Jinka TR, T?ien? ivind, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A1 receptors. J Neurosci. 2011;31: 10752–10758. 10.1523/JNEUROSCI.1240-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krumschnabel G, Biasi C, Wieser W. Action of adenosine on energetics, protein synthesis and K(+) homeostasis in teleost hepatocytes. J Exp Biol. 2000;203: 2657–2665. [DOI] [PubMed] [Google Scholar]
- 29. Pék M, Lutz PL. Role for adenosine in channel arrest in the anoxic turtle brain. J Exp Biol. 1997;200: 1913–1917. [DOI] [PubMed] [Google Scholar]
- 30. Cortés D, Guinzberg R, Villalobos-Molina R, Piña E. Evidence that endogenous inosine and adenosine-mediated hyperglycaemia during ischaemia–reperfusion through A3 adenosine receptors. Auton Autacoid Pharmacol. 2009;29: 157–164. 10.1111/j.1474-8665.2009.00443.x [DOI] [PubMed] [Google Scholar]
- 31. Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med. 2000;28: 3198–3202. [DOI] [PubMed] [Google Scholar]
- 32. Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616: 7–15. 10.1016/j.ejphar.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 33. Dolezal T, Dolezelova E, Zurovec M, Bryant PJ. A role for adenosine deaminase in Drosophila larval development. PLoS Biol. 2005;3: e201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dolezelova E, Nothacker H-P, Civelli O, Bryant PJ, Zurovec M. A Drosophila adenosine receptor activates cAMP and calcium signaling. Insect Biochem Mol Biol. 2007;37: 318–329. [DOI] [PubMed] [Google Scholar]
- 35. Fenckova M, Hobizalova R, Fric ZF, Dolezal T. Functional characterization of ecto-5’-nucleotidases and apyrases in Drosophila melanogaster. Insect Biochem Mol Biol. 2011;41: 956–967. 10.1016/j.ibmb.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 36. Knight D, Harvey PJ, Iliadi KG, Klose MK, Iliadi N, Dolezelova E, et al. Equilibrative nucleoside transporter 2 regulates associative learning and synaptic function in Drosophila. J Neurosci Off J Soc Neurosci. 2010;30: 5047–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zurovec M, Dolezal T, Gazi M, Pavlova E, Bryant PJ. Adenosine deaminase-related growth factors stimulate cell proliferation in Drosophila by depleting extracellular adenosine. Proc Natl Acad Sci U S A. 2002;99: 4403–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zuberova M, Fenckova M, Simek P, Janeckova L, Dolezal T. Increased extracellular adenosine in Drosophila that are deficient in adenosine deaminase activates a release of energy stores leading to wasting and death. Dis Model Mech. 2010;3: 773–784. 10.1242/dmm.005389 [DOI] [PubMed] [Google Scholar]
- 39. Novakova M, Dolezal T. Expression of Drosophila adenosine deaminase in immune cells during inflammatory response. PLoS ONE. 2011;6: e17741 10.1371/journal.pone.0017741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keebaugh ES, Schlenke TA. Insights from natural host–parasite interactions: The Drosophila model. Dev Comp Immunol. 2014;42: 111–123. 10.1016/j.dci.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 2010;346: 310–319. 10.1016/j.ydbio.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 42. Kacsoh BZ, Schlenke TA. High Hemocyte Load Is Associated with Increased Resistance against Parasitoids in Drosophila suzukii, a Relative of D. melanogaster. PLoS ONE. 2012;7: e34721 10.1371/journal.pone.0034721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorrentino RP, Melk JP, Govind S. Genetic Analysis of Contributions of Dorsal Group and JAK-Stat92E Pathway Genes to Larval Hemocyte Concentration and the Egg Encapsulation Response in Drosophila. Genetics. 2004;166: 1343–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reyes-DelaTorre A, Teresa M, Rafael J. Carbohydrate Metabolism in Drosophila: Reliance on the Disaccharide Trehalose. In: Chang C-F, editor. Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology. InTech; 2012. Available: http://www.intechopen.com/books/carbohydrates-comprehensive-studies-on-glycobiology-and-glycotechnology/carbohydrate-metabolism-in-drosophila-reliance-on-the-disaccharide-trehalose
- 45. Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009;7: e1000150–e1000150. 10.1371/journal.pbio.1000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Machado J, Abdulla P, Hanna WJB, Hilliker AJ, Coe IR. Genomic analysis of nucleoside transporters in Diptera and functional characterization of DmENT2, a Drosophila equilibrative nucleoside transporter. Physiol Genomics. 2007;28: 337–347. [DOI] [PubMed] [Google Scholar]
- 47. Brand a H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Dev Camb Engl. 1993;118: 401–415. [DOI] [PubMed] [Google Scholar]
- 48. Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, et al. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28: 143–180. [DOI] [PubMed] [Google Scholar]
- 49. Straub RH. Insulin resistance, selfish brain, and selfish immune system: an evolutionarily positively selected program used in chronic inflammatory diseases. Arthritis Res Ther. 2014;16: S4 10.1186/ar4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, et al. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147: 1589–1600. 10.1016/j.cell.2011.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morin-Poulard I, Vincent A, Crozatier M. The Drosophila JAK-STAT pathway in blood cell formation and immunity. JAK-STAT. 2013;2: e25700 10.4161/jkst.25700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Makki R, Meister M, Pennetier D, Ubeda J-M, Braun A, Daburon V, et al. A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol. 2010;8: e1000441–e1000441. 10.1371/journal.pbio.1000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andersen S. The roles of insulin and hyperglycemia in sepsis pathogenesis. J Leukoc Biol. 2004;75: 413–421. [DOI] [PubMed] [Google Scholar]
- 54. Faulhaber-Walter R, Jou W, Mizel D, Li L, Zhang J, Kim SM, et al. Impaired Glucose Tolerance in the Absence of Adenosine A1 Receptor Signaling. Diabetes. 2011;60: 2578–2587. 10.2337/db11-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, et al. Links Between Insulin Resistance, Adenosine A2B Receptors, and Inflammatory Markers in Mice and Humans. Diabetes. 2011;60: 669–679. 10.2337/db10-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Németh ZH, Csóka B, Wilmanski J, Xu D, Lu Q, Ledent C, et al. Adenosine A2A Receptor Inactivation Increases Survival in Polymicrobial Sepsis. J Immunol. 2006;176: 5616–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sullivan GW, Fang G, Linden J, Scheld WM. A2A Adenosine Receptor Activation Improves Survival in Mouse Models of Endotoxemia and Sepsis. J Infect Dis. 2004;189: 1897–1904. [DOI] [PubMed] [Google Scholar]
- 58. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37: 911–917. [DOI] [PubMed] [Google Scholar]
- 59. Tennessen JM, Barry W, Cox J, Thummel CS. Methods for studying metabolism in Drosophila. Methods. 2014;68: 105–115. 10.1016/j.ymeth.2014.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig 1 —data.pzfx: Immune response to parasitoid wasp intrusion. Fig 2 —data.pzfx: Metabolic changes during immune response in w flies. Fig 3 —data.pzfx: Gene expression during immune response of w larvae measured by q-PCR. Fig 4 —data.pzfx: Effects of blocking signaling through adenosine receptor (adoR) on immune response. Fig 5 —data.pzfx: Metabolic changes and developmental effects of AdoR deficiency. Fig 6 —data.pzfx: Effects of blocking adenosine transport in immune cells by ENT2 RNAi. S2 Fig—data.pzfx: Statistical analysis of metabolite changes. S3 Fig—data.pzfx: Gene expression analysis of glycolytic and citrate cycle genes in fat body by q-PCR. S4 Fig—data.pzfx: Gene expression analysis of glycolytic and citrate cycle genes in circulating hemocytes by q-PCR. S5 Fig—data.pzfx: Gene expression analysis of glycolytic and citrate cycle genes in lymph gland by q-PCR. S7 Fig—data.pzfx: Recognition of parasitoid wasp egg by plasmatocytes is not affected by adoR. S8 Fig—data.pzfx: Total absorption of 14C from food. S9–S10 Fig—data.pzfx: Comparison of relative 14C-tissue distribution between infected and uninfected larvae and between w and adoR. S11 Fig—data.pzfx: q-PCR expression analysis of genes involved in transport and metabolism of glucose and trehalose in w and adoR. S12 Fig—data.pzfx: Number of lamellocytes in flies with knocked-down equilibrative nucleoside transporters ENT1 and ENT2 in different tissues. S14 Fig—data.pzfx: Relative 14C incorporation into macromolecules in w and Srp>ENT2-RNAi at 6 hpi. S15 Fig—data.pzfx: Expression analysis of genes involved in regulation of lamellocytes differentiation by q-PCR.
(ZIP)
Top timescale—sampling and treatment description for experiments with 14C-labeled glucose. Middle timescale—sample collection and treatment for experiments characterizing reaction to infection. Bottom timescale—sample collection for experiments characterizing metabolites.
(TIF)
A) Statistical analysis of infection-induced changes in circulating trehalose between infection and control in particular time points (upper table) and between different time points either in control or infection (lower table); tested by two-way ANOVA, B) comparison of incorporation of 14C into macromolecules (saccharides, lipids, and proteins), and C) into three distinguished processes (development and growth, cellular immunity, and circulation) in w and adoR; tested by two-way ANOVA. Uninfected individuals marked as CON (grey columns), infected individuals marked as INF (black columns). Graphs show mean values ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
Infection-induced difference in gene expression was analyzed in w and adoR, 6 and 18 hpi. Uninfected individuals are represented by grey columns (CON), infected individuals by black columns (INF). Graphs show mean values relative to Rp49 ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005); tested by one-way ANOVA. Gene symbols and the corresponding genes can be found in S1 Table.
(TIF)
Infection-induced difference in gene expression was analyzed in w and adoR, 6 and 18 hpi. Uninfected individuals are represented by grey columns (CON), infected individuals by black columns (INF). Graphs show mean values relative to Rp49 ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005); tested by one-way ANOVA. Gene symbols and the corresponding genes can be found in S1 Table.
(TIF)
Infection-induced difference in gene expression was analyzed in w and adoR, 6 and 18 hpi. Uninfected individuals are represented by grey columns (CON), infected individuals by black columns (INF). Graphs show mean values relative to Rp49 ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005); tested by one-way ANOVA. Gene symbols and the corresponding genes can be found in S1 Table.
(TIF)
Comparison of infection-induced differences in gene expression between w and adoR in three different tissues (hemocytes, lymph gland, and fat body) and two different time points postinfection (6 and 18 hpi). Green squares—increased expression, red squares—decreased expression, grey squares—no significant difference, white squares—not analyzed. Level of significance p < 0.05; one-way ANOVA.
(TIF)
(A) Percentage of eggs with certain number of plasmatocytes (none, 1–10, 10–20, or >20) attached to their surface within the first 2 hpi and 4–6 hpi in w and adoR mutant larvae. (B) Examples of attached Hml>GFP-labeled hemocytes (green fluorescence) to parasitoid wasp egg within the first 2 hpi and 4–6 hpi in w and adoR mutant larvae.
(TIF)
Larvae were fed 14C-glucose (in blue dye-labeled diet) for 20 min and then transferred to normal diet for 5 h in which they absorbed 14C-glucose and cleared their guts. They were then homogenized to analyze how much 14C-glucose they absorbed. There is no difference in absorption between control w and control adoR or infected w and infected adoR both at 6 and 18 hpi (labeled NS for not significant). Interestingly, infected larvae (both w and adoR) absorbed less 14C than control larvae indicating anorexia upon infection. Graph shows uninfected w (grey columns), infected w (black columns), uninfected adoR (pink columns), and infected adoR (red columns) mean values of disintegration of 14C per minute (dpm) per sample ± SEM of three independent experiments, tested by one-way ANOVA. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
This figure serves as an alternative for Fig 5B to visualize statistical significance. Compared values: uninfected w (grey columns) with infected w (black columns) and uninfected adoR (pink columns) with infected adoR (red columns). Graph shows mean values ± SEM of three independent experiments. Tested by one-way ANOVA with Arc-Sin transformation. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
This figure serves as an alternative for Fig 5B to visualize statistical significance. Compared values: uninfected w (grey columns) with uninfected adoR (pink columns) and infected w (black columns) with infected adoR (red columns). Graph shows mean values ± SEM of three independent experiments. Tested by one-way ANOVA with Arc-Sin transformation. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005).
(TIF)
Graphs display infection-induced differences in expression level of Trehalose transporter TreT1-1, Glucose transporter 1 (Glut1), Glycogen synthase (Gsyn), and Glycogen phosphorylase (Gps) in fat body, lymph gland, and circulating hemocytes at 6 and 18 hpi. Uninfected individuals marked as CON (grey columns), infected individuals marked as INF (black columns). Graph shows mean values ±SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005; ns for nonsignificant difference); tested by one-way ANOVA.
(TIF)
RNAi was induced by driving UAS-Ent1-RNAi (VDRC ID 109885) and UAS-Ent2-RNAi (VDRC ID 100464) by various Gal4 drivers: Srp-Gal4 expressed in all cells of hematopoietic lineage and fat body, Upd3-Gal4 and Hml-Gal4 in differentiated hemocytes and C7-Gal4 in fat body. Lamellocytes were counted based on morphology using DIC at 24 hpi. Only combination of Srp>Ent2-RNAi significantly decreased lamellocytes. Results were tested by one-way ANOVA, each point in graph represents number of lamellocytes in one individual larva. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005; NS for nonsignificant difference).
(TIF)
Srp-Gal4 driver expression was visualized by crossing to UAS-GFP. Strong expression was detected in all cells of hematopoietic lineage as demonstrated by the GFP fluorescence in circulating hemocytes and all cells in the lymph gland. The expression was also detected in fat body but it was undetectable in the brain besides weak expression in nerve cords. Left panels show DIC image corresponding to GFP fluorescence images on right.
(TIF)
Comparison of incorporation of 14C into macromolecules (saccharides, lipids, and proteins) tested by two-way ANOVA. Uninfected individuals are marked as control (grey columns), infected individuals are marked as infected (black columns). Graphs show mean values ± SEM of three independent experiments. Asterisks show statistical significance (*<0.05; **<0.005; ***<0.0005; ns for not significant).
(TIF)
RNA was isolated from dissected lymph glands at 6 hpi of control (grey) and infected (black) larvae. Graphs show the reciprocal changes in expression of Jak-Stat receptor Domeless and its negative coreceptor Latran and turning off Unpaired3 (Upd3) upon infection. This hallmark of induction of lamellocyte differentiation does not differ in adoR or Srp>ENT2-RNAi compared to w. Graph shows mean values ± SEM of three independent experiments. Differences were tested by one-way ANOVA; results shown below the graphs—ns = not significant; *<0.05; **<0.005; ***<0.0005; ****<0.00005).
(TIF)
(A) Expression of MSNF9>GFP (green) together with plasmatocytes-specific P1 marker (red) in the lymph gland upon infection at 12 and 18 hpi. Both w and adoR express the lamellocyte marker indicating that adoR is able to differentiate lamellocytes but there is usually less MSNF9>GFP signal in adoR at 12 hpi. While w sometimes already releases lamellocytes into circulation at 18 hpi (as demonstrated by disintegrated lymph gland in DIC picture), adoR has lymph gland still compact at 18 hpi, but with increasing number of MSNF9>GFP positive cells further demonstrating ability of adoR to differentiate lamellocytes but with lower speed. Top—DIC, bottom—fluorescence confocal image. (B) MSNF9>GFP positive lamellocytes in circulation at 27 hpi are present in both w and adoR and their morphology is indistinguishable. Fluorescence and DIC-combined micrographs.
(TIF)
Morphology and zonation of the lymph gland in 72-h-old larvae (corresponding to time of infection) was checked by expression of medullary zone-specific Dome>GFP marker (prohemocyte containing zone; green) and differentiated plasmatocyte-specific P1 marker for cortical zone (red) in w and adoR. Only P1 marker was used in Srp>ENT2-RNAi and DAPI (nuclear staining) for overall morphology to define medullary zone by the absence of P1. In all three genotypes, the zonation and morphology was comparable for multiple samples indicating that there is no gross effect of the used genetic manipulations on the lymph gland development prior to infection.
(TIF)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.