Figure 1.
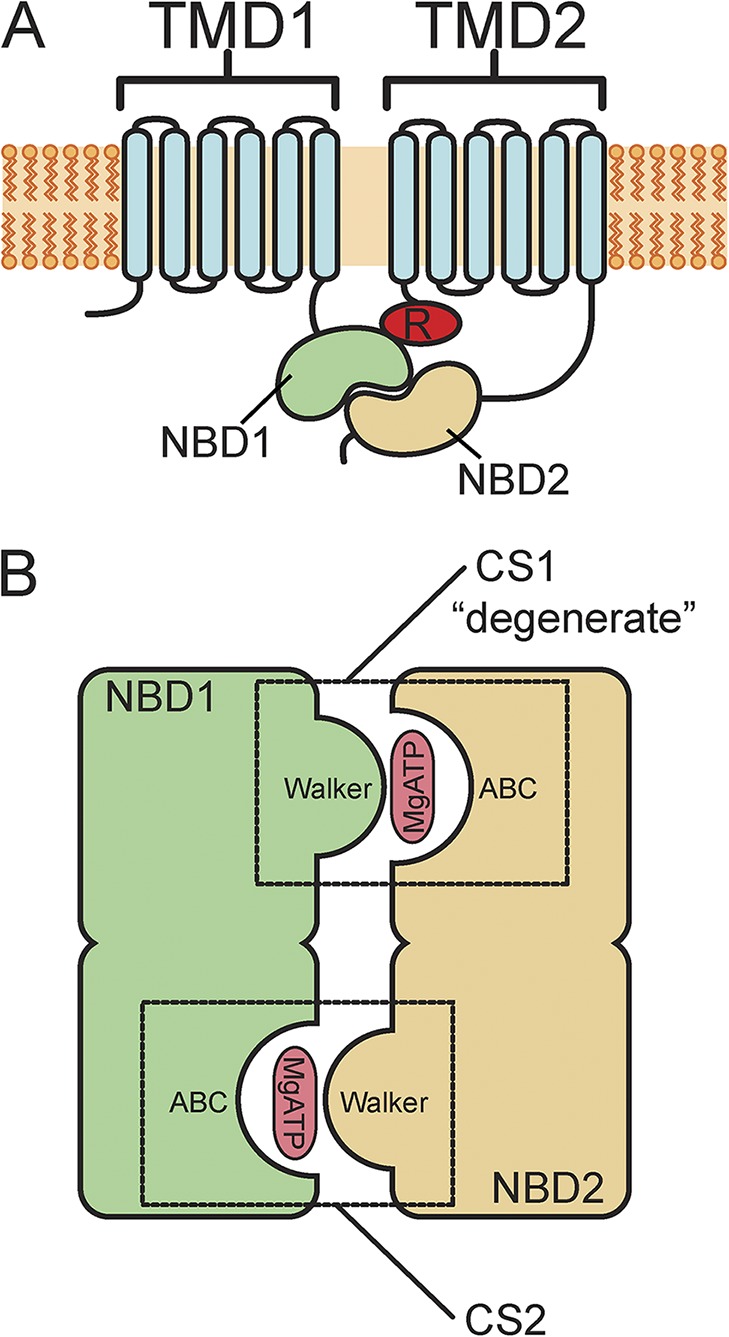
Schematic structure of CFTR and its NBDs. (A) Membrane topology of CFTR channels. CFTR consists of a single polypeptide chain with intracellular amino and carboxyl termini. There are two transmembrane domains (TMD1 and TMD2) and two NBDs (NBD1 and NBD2). There is also a regulatory domain (R) between NBD1 and TMD2. (B) Schematic of the NBDs (NBD1 and NBD2). NBD1 and NBD2 form a pseudo-twofold dimer with MgATP bound in between. The two composite nucleotide-binding sites (CS1 and CS2) are formed in part from the Walker A and B motifs (Walker) of one NBD and the ABC signature sequence (ABC) of the other. CS1, formed from the Walker groups of NBD1 and the ABC signature sequence of NBD2, is labeled “degenerate,” as it is catalytically incompetent.
