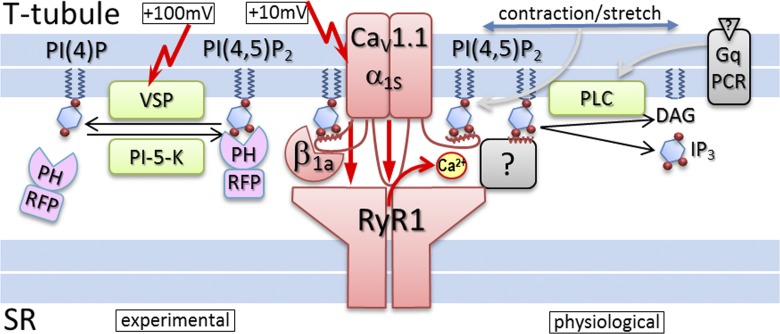
Figure 1.
PI(4,5)2 modulation of skeletal muscle EC coupling. In the center, the EC-coupling complex (red)—composed of the L-type calcium channel subunits CaV1.1 α1s and β1a in the T-tubule and the RyR1 calcium release channel in the SR—is displayed. The experimental tools used by Berthier et al. (2015) to deplete PI(4,5)P2 (VSP) and monitor PI(4,5)P2 depletion (PH-RFP, PLCδ1PH-mRFP) are shown on the left. Note that EC coupling is activated at membrane potentials of ≥10 mV, whereas activation of VSP requires repetitive depolarization to ≥100 mV. On the right, physiological signaling pathways that mediate PI(4,5)P2 depletion are shown. It is also possible that PI(4,5)P2 modulation of the EC-coupling complex is caused by the physical force of membrane stretch during contraction. PI(4,5)P2 might interact with binding sites on CaV1.1, like that in close proximity to the binding site for β1a, and thereby facilitate EC coupling. Alternatively, PI(4,5)P2 could interact with other triad proteins spanning the gap between the T-tubule membrane and the RyR1 to facilitate SR calcium release without modulating CaV1.1 calcium currents.